tert-Butyl hydroperoxide
tert-Butyl hydroperoxide (tBuOOH) is an organic peroxide widely used in a variety of oxidation processes, for example Sharpless epoxidation.[3] It is normally supplied as a 69–70% aqueous solution.
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
2-Methylpropane-2-peroxol[1] | |||
| Systematic IUPAC name
tert-Butyl hydroperoxide | |||
| Identifiers | |||
3D model (JSmol) |
|||
| Abbreviations | TBHP | ||
| 1098280 | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.000.833 | ||
| EC Number |
| ||
| MeSH | tert-Butylhydroperoxide | ||
PubChem CID |
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 3109 | ||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C4H10O2 | |||
| Molar mass | 90.122 g·mol−1 | ||
| Appearance | Colorless liquid | ||
| Density | 0.935 g/mL | ||
| Melting point | −3 °C (27 °F; 270 K) | ||
| Boiling point | 37 °C (99 °F; 310 K) at 2.0 kPa | ||
| miscible | |||
| log P | 1.23 | ||
| Acidity (pKa) | 12.69 | ||
| Basicity (pKb) | 1.31 | ||
Refractive index (nD) |
1.3870 | ||
| Thermochemistry | |||
Std enthalpy of formation (ΔfH⦵298) |
−294±5 kJ/mol | ||
Std enthalpy of combustion (ΔcH⦵298) |
2.710±0.005 MJ/mol | ||
| Hazards | |||
| Safety data sheet | |||
| GHS pictograms |      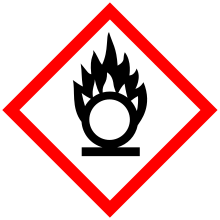 | ||
| GHS Signal word | Danger | ||
GHS hazard statements |
H226, H242, H302, H311, H314, H317, H331, H341, H411 | ||
| P220, P261, P273, P280, P305+351+338, P310 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | 43 °C (109 °F; 316 K) | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Application
Industrially, tert-butyl hydroperoxide is used as a radical polymerization initiator. For example, its reaction with propene yields propylene oxide and the byproduct t-butanol which can dehydrate to isobutene and convert to MTBE.
Synthesis and production
Many synthetic routes are available, including:[4]
- Reaction of hydrogen peroxide with isobutylene or tert-butyl alcohol in the presence of sulfuric acid
- Auto-oxidation of isobutane with oxygen
Safety
tert-butyl hydroperoxide is an exceptionally dangerous chemical that is highly reactive, flammable and toxic. It is corrosive to skin and mucous membranes and causes respiratory distress when inhaled. [5]
A solution of tert-butyl hydroperoxide and water with a concentration of greater than 90% is forbidden to be shipped according to US Department of Transportation Hazardous Materials Table 49 CFR 172.101.
In some sources it also has an NFPA 704 rating of 4 for health, 4 for flammability, 4 for reactivity and is a potent oxidant,[6] however other sources claim lower ratings of 3-2-2 or 1-4-4.[7][8]
See also
References
- "IUPAC Complete Draft 2004" (PDF).
- Cameo Chemicals, reference for NFPA values.
- tert-butyl hydroperoxide at Organic Chemistry Portal
- Jose Sanchez; Terry N. Myers. "Peroxides and Peroxide Compounds, Organic Peroxides". Kirk‑Othmer Encyclopedia of Chemical Technology. Wiley-VCH Verlag GmbH & Co. doi:10.1002/0471238961.1518070119011403.a01.
- Sigma Aldrich MSDS
- "TERT-BUTYL HYDROPEROXIDE" at CAMEO Chemicals NOAA
- tert-BUTYL HYDROPEROXIDE at Chemicalland21
- tert-Butyl hydroperoxide at http://environmentalchemistry.com