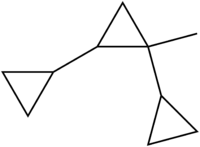
Syntin
Syntin is a hydrocarbon with the molecular formula C10H16 used as a rocket fuel. It is a mixture of four stereoisomers (see below). It has a density of 0.851 g/mL, and a boiling point of 158 °C. Due to the presence of three strained cyclopropane rings, the molecule has a highly positive enthalpy of formation: ΔfH°(l)= 133 kJ/mol (980 kJ/kg, the average value for the isomeric mixture), bringing additional energy into the combustion process. It has advantages over the traditional hydrocarbon fuels, such as RP-1, due to higher density, lower viscosity and higher specific heat of oxidation.
 | |
| Names | |
|---|---|
| IUPAC name
1′-Methyl-1,1′:2′,1′′-tercyclopropane | |
| Other names
1-Methyl-1,2-dicyclopropylcyclopropane; Sintin; Synthin; Tsycklin | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C10H16 | |
| Molar mass | 136.238 g·mol−1 |
| Density | 0.851 g/mL |
| Boiling point | 158 °C (316 °F; 431 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Syntin was used in the Soviet Union and later Russia in 1980s-1990s as fuel for the Soyuz-U2 rocket. It was first synthesized in the USSR in the 1960s and brought to mass production in the 1970s. It was prepared in a multi-step synthetic process from easily obtained acetylcyclopropane:
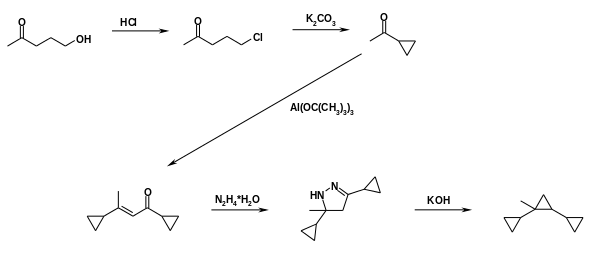 Syntin synthesis 01
Syntin synthesis 01
After dissolution of the USSR, the production of this fuel was halted due to the expense of the synthesis.
Stereoisomers
Syntin is a molecule with two stereocenters at the central cyclopropane ring. Thus, the following four stereoisomers may exist:
 Four Syntin Stereoisomers
Four Syntin Stereoisomers
In practice, the fuel has been used as mixture of all four stereoisomers.
See also
References
- A. P. Mesheheryakov, V. G. Glukhovtsev, A. D. Petrov, “Synthesis of 1-methyl-1,2-dicyclopropylcyclopropane”, Doklady Akademii Nauk SSSR, 1960, 130, 779-81.
- Yu. P. Semenov, B. A. Sokolov, S. P. Chernykh, A. A. Grigor'ev, O. M. Nefedov, N. N. Istomin, G. M. Shirshov, “Multiple strained-ring alkane as high-performance liquid rocket fuel”, RU 2233385, C2 20040727.
- T. Edwards, “Liquid Fuels and Propellants for Aerospace Propulsion: 1903-2003”, Journal of Propulsion and Power, 2003, 19(6), 1089-1107.
- V. Azov, D. Vorontsov, "The last battle of hydrocarbons?", Novosti Kosmonavtiki, 2008, 18, No. 2 (301), 44-46.