Synteny
In classical genetics, synteny describes the physical co-localization of genetic loci on the same chromosome within an individual or species. Today, however, biologists usually refer to synteny as the conservation of blocks of order within two sets of chromosomes that are being compared with each other. This concept can also be referred to as shared synteny.
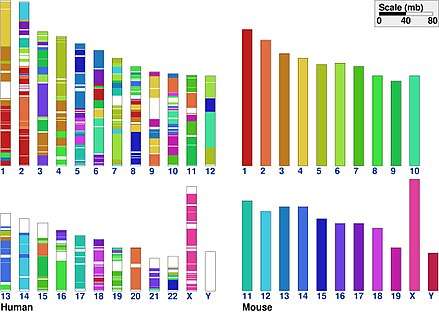
The classical concept is related to genetic linkage: Linkage between two loci is established by the observation of lower-than-expected recombination frequencies between them. In contrast, any loci on the same chromosome are by definition syntenic, even if their recombination frequency cannot be distinguished from unlinked loci by practical experiments. Thus, in theory, all linked loci are syntenic, but not all syntenic loci are necessarily linked. Similarly, in genomics, the genetic loci on a chromosome are syntenic regardless of whether this relationship can be established by experimental methods such as DNA sequencing/assembly, genome walking, physical localization or hap-mapping.
Students of genetics employ the term synteny to describe the situation in which two genetic loci have been assigned to the same chromosome but still may be separated by a large enough distance in map units that genetic linkage has not been demonstrated.
The Encyclopædia Britannica gives the following description of synteny:[2]
Genomic sequencing and mapping have enabled comparison of the general structures of genomes of many different species. The general finding is that organisms of relatively recent divergence show similar blocks of genes in the same relative positions in the genome. This situation is called synteny, translated roughly as possessing common chromosome sequences. For example, many of the genes of humans are syntenic with those of other mammals—not only apes but also cows, mice, and so on. Study of synteny can show how the genome is cut and pasted in the course of evolution.
Etymology
Synteny is a neologism meaning "on the same ribbon"; Greek: σύν, syn = along with + ταινία, tainiā = band, referring to the same order of genes on two (homologous) strings of DNA (or chromosomes).
Shared synteny
Shared synteny (also known as conserved synteny) describes preserved co-localization of genes on chromosomes of different species. During evolution, rearrangements to the genome such as chromosome translocations may separate two loci, resulting in the loss of synteny between them. Conversely, translocations can also join two previously separate pieces of chromosomes together, resulting in a gain of synteny between loci. Stronger-than-expected shared synteny can reflect selection for functional relationships between syntenic genes, such as combinations of alleles that are advantageous when inherited together, or shared regulatory mechanisms.[3]
The term is sometimes also used to describe preservation of the precise order of genes on a chromosome passed down from a common ancestor,[4][5][6][7] although many geneticists reject this use of the term.[8]
The analysis of synteny in the gene order sense has several applications in genomics. Shared synteny is one of the most reliable criteria for establishing the orthology of genomic regions in different species. Additionally, exceptional conservation of synteny can reflect important functional relationships between genes. For example, the order of genes in the "Hox cluster", which are key determinants of the animal body plan and which interact with each other in critical ways, is essentially preserved throughout the animal kingdom.[9]
Synteny is widely used in studying complex genomes, as comparative genomics allows the presence and possibly function of genes in a simpler, model organism to infer those in a more complex one. For example, wheat has a very large, complex genome which is difficult to study. In 1994 research from the John Innes Centre in England and the National Institute of Agrobiological Research in Japan demonstrated that the much smaller rice genome had a similar structure and gene order to that of wheat.[10] Further study found that many cereals are syntenic [11] and thus plants such as rice or the grass Brachypodium could be used as a model to find genes or genetic markers of interest which could be used in wheat breeding and research. In this context, synteny was also essential in identifying a highly important region in wheat, the Ph1 locus involved in genome stability and fertility, which was located using information from syntenic regions in rice and Brachypodium.[12]
Synteny is also widely used in microbial genomics. In Rhizobiales and Enterobacteriales, syntenic genes encode a large number of essential cell functions and represent a high level of functional relationships.[13]
Patterns of shared synteny or synteny breaks can also be used as characters to infer the phylogenetic relationships among several species, and even to infer the genome organization of extinct ancestral species. A qualitative distinction is sometimes drawn between macrosynteny, preservation of synteny in large portions of a chromosome, and microsynteny, preservation of synteny for only a few genes at a time.
Computational detection
Shared synteny between different species can be inferred from their genomic sequences. This is typically done using a version of the MCScan algorithm, which finds syntenic blocks between species by comparing their homologous genes and looking for common patterns of collinearity on a chromosomal or contig scale. Homologies are usually determined on the basis of high bit score BLAST hits that occur between multiple genomes. From here, dynamic programming is used to select the best scoring path of shared homologous genes between species, taking into account potential gene loss and gain which may have occurred in the species' evolutionary histories.[14]
References
- Sinha, Amit U.; Meller, Jaroslaw (2007-03-08). "Cinteny: flexible analysis and visualization of synteny and genome rearrangements in multiple organisms". BMC Bioinformatics. 8: 82. doi:10.1186/1471-2105-8-82. ISSN 1471-2105. PMC 1821339. PMID 17343765.
- heredity (genetics) : Microevolution - Britannica Online Encyclopedia
- Moreno-Hagelsieb G, Treviño V, Pérez-Rueda E, Smith TF, Collado-Vides J (2001). "Transcription unit conservation in the three domains of life: a perspective from Escherichia coli". Trends in Genetics. 17 (4): 175–177. doi:10.1016/S0168-9525(01)02241-7. PMID 11275307.
- Engström PG, Ho Sui SJ, Drivenes O, Becker TS, Lenhard B (2007). "Genomic regulatory blocks underlie extensive microsynteny conservation in insects". Genome Res. 17 (12): 1898–908. doi:10.1101/gr.6669607. PMC 2099597. PMID 17989259.
- Heger A, Ponting CP (2007). "Evolutionary rate analyses of orthologs and paralogs from 12 Drosophila genomes". Genome Res. 17 (12): 1837–49. doi:10.1101/gr.6249707. PMC 2099592. PMID 17989258.
- Poyatos JF, Hurst LD (2007). "The determinants of gene order conservation in yeasts". Genome Biol. 8 (11): R233. doi:10.1186/gb-2007-8-11-r233. PMC 2258174. PMID 17983469.
- Dawson DA, Akesson M, Burke T, Pemberton JM, Slate J, Hansson B (2007). "Gene order and recombination rate in homologous chromosome regions of the chicken and a passerine bird". Mol. Biol. Evol. 24 (7): 1537–52. doi:10.1093/molbev/msm071. PMID 17434902.
- Passarge E, Horsthemke B, Farber RA (December 1999). "Incorrect use of the term synteny". Nature Genetics. 23 (4): 387. doi:10.1038/70486. PMID 10581019.
- Amores A, Force A, Yan YL, Joly L, Amemiya C, Fritz A, Ho RK, Langeland J, Prince V, Wang YL, Westerfield M, Ekker M, Postlethwait JH (November 1998). "Zebrafish hox clusters and vertebrate genome evolution". Science. 282 (5394): 1711–4. doi:10.1126/science.282.5394.1711. PMID 9831563.
- Kurata N, Moore G, Nagamura Y, Foote T, Yano M, Minobe Y, Gale M (1994). "Conservation of genome structure between rice and wheat". Nature Biotechnology. 12 (3): 276–278. doi:10.1038/nbt0394-276.
- Moore G, Devos KM, Wang Z, Gale MD (July 1995). "Cereal genome evolution. Grasses, line up and form a circle". Current Biology. 5 (7): 737–9. doi:10.1016/S0960-9822(95)00148-5. PMID 7583118.
- Griffiths S, Sharp R, Foote TN, Bertin I, Wanous M, Reader S, Colas I, Moore G (February 2006). "Molecular characterization of Ph1 as a major chromosome pairing locus in polyploid wheat". Nature. 439 (7077): 749–52. doi:10.1038/nature04434. PMID 16467840.
- Guerrero, G; Peralta, H; Aguilar, A; Díaz, R; Villalobos, MA; Medrano-Soto, A; Mora, J (17 October 2005). "Evolutionary, structural and functional relationships revealed by comparative analysis of syntenic genes in Rhizobiales". BMC Evolutionary Biology. 5: 55. doi:10.1186/1471-2148-5-55. PMC 1276791. PMID 16229745.
- Wang, Y; Tang, H; Debarry, JD; Tan, X; Li, J; Wang, X; Lee, TH; Jin, H; Marler, B; Guo, H; Kissinger, JC; Paterson, AH (April 2012). "MCScanX: a toolkit for detection and evolutionary analysis of gene synteny and collinearity". Nucleic Acids Research. 40 (7): e49. doi:10.1093/nar/gkr1293. PMC 3326336. PMID 22217600.
External links
- ACT (Artemis Comparison Tool) — Probably the most used synteny software program used in comparative genomics.
- Comparative Maps NIH's National Library of Medicine NCBI link to Gene Homology resources, and Comparative Chromosome Maps of the Human, Mouse, and Rat.
- Graham Moore group research page - cereal genomics More information on synteny and its use in comparative cereal genomics.
- NCBI Home Page NIH's National Library of Medicine NCBI (National Center for Biotechnology Information) link to a tremendous number of resources.
- SimpleSynteny A free browser-based tool to compare and visualize microsynteny across multiple genomes for a set of genes.
- Synteny server Server for Synteny Identification and Analysis of Genome Rearrangement—the Identification of synteny and calculating reversal distances.
- PlantSyntenyViewer A web based visualisation tool allowing to navigate on genomes and visualizing the Synteny conservation among several datasets (cereals, dicotyledons, animals, a Wheat-based one...)