Sulfiram
Sulfiram (INN) or monosulfiram, trade name Tetmosol, is an ectoparasiticide used in the treatment and prevention of scabies.[1] It is usually sold as a solution or medicated soap, sometimes in combination with benzyl benzoate.
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Topical |
| ATCvet code | |
| Pharmacokinetic data | |
| Bioavailability | Very low |
| Excretion | Renal, unchanged |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.002.171 |
| Chemical and physical data | |
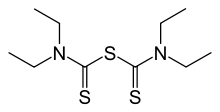
| Formula | C10H20N2S3 |
| Molar mass | 264.46 g·mol−1 |
Sulfiram is now rarely used, but, as of 2015, is still available in Brazil, India, and South Africa (as monotherapy).[1]
Adverse effects
Dizziness, headache, fatigue and erythematous rash may occur.[2] A single case of toxic epidermal necrolysis was reported in 1968.[3]
Sulfiram is structurally related to disulfiram (Antabuse), and readily converts to disulfiram when exposed to light. Like disulfiram, it can produce an unpleasant reaction when consumed with alcohol.[1][4]
gollark: Also, I think using the numbering system is kind of a bad idea.
gollark: You can probably shorten it a bit.
gollark: I don't know.
gollark: Okay, I can agree with that.
gollark: <@330678593904443393> How's this:"Add a rule section: 13 PropertiesA property is a value associated with each player, similarly to quantities but without type restrictions.By default any unique property added to the game: • applies to all players, • is instatiated at "" (the empty string)"
References
- Sweetman, Sean C., ed. (2009). "Pesticides and repellents". Martindale: the complete drug reference (36th ed.). London: Pharmaceutical Press. p. 2050. ISBN 978-0-85369-840-1.
- [No authors listed] (2009). "Sarfiram - Bula". Bulário de Remédios Comerciais (in Portuguese). MedicinaNET. Retrieved 2010-08-11.
- Copeman PW (March 1968). "Toxic epidermal necrolysis caused by skin hypersensitivity to monosulfiram". British Medical Journal. 1 (5592): 623–4. doi:10.1136/bmj.1.5592.623. PMC 1985336. PMID 5637574.
- Mays DC, Nelson AN, Benson LM, Johnson KL, Naylor S, Lipsky JJ (November 1994). "Photolysis of sulfiram: a mechanism for its disulfiram-like reaction". Biochemical Pharmacology. 48 (10): 1917–25. doi:10.1016/0006-2952(94)90590-8. PMID 7986203.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.