Sulfinylamine
Sulfinylamines are organosulfur compounds with the formula RNSO where R = an organic substituent. These compounds are, formally speaking, derivatives of HN=S=O, i.e. analogues of sulfur dioxide and of sulfur diimide. A common example is N-sulfinylaniline. Sulfinyl amines are dienophile.[1] They undergo [2+2] cycloaddition to ketenes.[2]
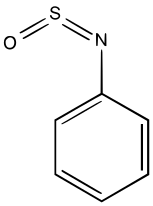
N-Sulfinylaniline is a common sulfinylamine.
According to X-ray crystallography, sulfinylamines have planar C-N=S=O cores with syn geometry.[3]
References
- Kresze, G.; Wucherpfennig, W., "Organic synthesis with imides of sulfur dioxide", Angew. Chem. Int. Ed. Engl. 1967, volume 6, 149-167. doi:10.1002/anie.196701491
- Heravi, Majid M.; Talaei, Bahareh (2014). Ketenes as Privileged Synthons in the Syntheses of Heterocyclic Compounds. Part 1. Advances in Heterocyclic Chemistry. 113. pp. 143–244. doi:10.1016/B978-0-12-800170-7.00004-3. ISBN 9780128001707.
- Romano, R.M.; Della Védova, C.O. (2000). "N-Sulfinylimine compounds, R–N=S=O: A chemistry family with strong temperament". Journal of Molecular Structure. 522 (1–3): 1–26. Bibcode:2000JMoSt.522....1R. doi:10.1016/S0022-2860(99)00453-6..
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.