Stork enamine alkylation
The Stork enamine alkylation, involves the addition of an enamine to an alpha, beta-unsaturated carbonyl acceptor in a process similar to the Michael reaction.[1] The product is then hydrolyzed by an aqueous acid to produce a 1,5-dicarbonyl compound.
The process:
- formation of an enamine from a ketone
- addition of the enamine to an alpha, beta-unsaturated aldehyde or ketone
- hydrolysis of the enamine back to a ketone
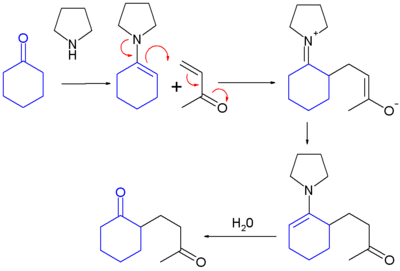
When the electrophile is an acyl halide, a 1,3-diketone is formed (Stork acylation).[2] The reaction is named after its inventor: Gilbert Stork.
Variations
In a special case of this reaction type it is also possible to alkylate ketones or aldehydes with alkyl halides as less reactive electrophiles:[3]

In this method a carbonyl compound is converted to an imine by alkylimino-de-oxo-bisubstitution with a primary amine. The imine is then reacted with a Grignard reagent to the corresponding magnesium salt to an intermediate capable of displacing a halide. Hydrolysis once again yields the alkylated ketone.
References
- McMurry, John (21 March 2003). Organic Chemistry (Hardcover) (6th ed.). Belmont, CA: Thomson-Brooks/Cole. ISBN 0-534-38999-6.
- March, Jerry (1985), Advanced Organic Chemistry: Reactions, Mechanisms, and Structure (3rd ed.), New York: Wiley, ISBN 0-471-85472-7
- A New Method for the Alkylation of Ketones and Aldehydes: the C-Alkylation of the Magnesium Salts of N-Substituted Imines Gilbert Stork and Susan R. Dowd J. Am. Chem. Soc.; 1963; 85(14) pp 2178–80; doi:10.1021/ja00897a040