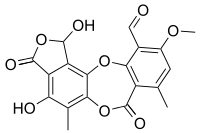
Stictic acid
Stictic acid is an aromatic organic compound, a product of secondary metabolism in some species of lichens.[1]
 | |
 | |
| Names | |
|---|---|
| Systematic IUPAC name
1,4-Dihydroxy-10-methoxy-5,8-dimethyl-3,7-dioxo-1,3-dihydro-7H-2,6,12-trioxabenzo[5,6]cyclohepta[1,2-e]indene-11-carbaldehyde | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.161.455 |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C19H14O9 | |
| Molar mass | 386.312 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Stictic acid is the subject of preliminary biomedical research. Stictic acid has cytotoxic and apoptotic effects in vitro.[2] Computational studies suggest stictic acid may also stimulate p53 reactivation.[3]
References
- Lohézic-Le Dévéhat, Françoise; Tomasi, Sophie; Elix, John A.; Bernard, Aurélie; Rouaud, Isabelle; Uriac, Philippe; Boustie, Joël (2007). "Stictic Acid Derivatives from the Lichen Usnea articulata and Their Antioxidant Activities". Journal of Natural Products. 70 (7): 1218–20. doi:10.1021/np070145k. PMID 17629329.
- Correché, ER; Enriz, RD; Piovano, M; Garbarino, J; Gómez-Lechón, MJ (2004). "Cytotoxic and apoptotic effects on hepatocytes of secondary metabolites obtained from lichens". Alternatives to Laboratory Animals. 32 (6): 605–15. PMID 15757498.
- Wassman, Christopher D.; Baronio, Roberta; Demir, Özlem; Wallentine, Brad D.; Chen, Chiung-Kuang; Hall, Linda V.; Salehi, Faezeh; Lin, Da-Wei; Chung, Benjamin P.; Wesley Hatfield, G.; Richard Chamberlin, A.; Luecke, Hartmut; Lathrop, Richard H.; Kaiser, Peter; Amaro, Rommie E. (2013). "Computational identification of a transiently open L1/S3 pocket for reactivation of mutant p53". Nature Communications. 4: 1407. doi:10.1038/ncomms2361. PMC 3562459. PMID 23360998.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.