Stannoxane
Stannoxane is a functional group in organotin chemistry with the connectivity SnIV-O-SnIV (IV indicates the oxidation state of tin). Aside from the oxide group, usually 3 or 4 other substituents are attached to tin. In aqueous or aquatic environments, most organotin compounds contain this group.[1]
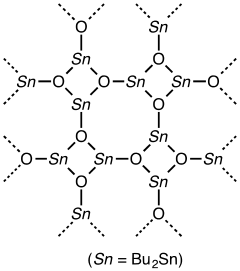 Dibutyltin oxide adopts a polymeric structure with many stannoxane linkages
Dibutyltin oxide adopts a polymeric structure with many stannoxane linkages
Synthesis and formation
Stannoxanes form upon hydrolysis of organotin halides. For example, hydrolysis of dibutyltin dichloride gives the tetratin compound {[Bu2ClSn]2O}2. The hydrolysis appears to proceed via organotin hydroxides. For example, the commercially important (C6H11)3SnOH converts at 200 °C into the distannoxane:
- 2 (C6H11)3SnOH → [(C6H11)3Sn]2O + H2O
The condensation process is proposed to occur via an associative mechanism, involving the dimer. Support for this associative mechanism is the finding that Me3SnOH exists in solution as the dimer (Me3Sn)2(μ-OH)2.
Reactivity
Indicative of the lability of the Sn-O bond, distannoxanes exchange with other distannoxanes:
- (R3Sn)2O + (R'3Sn)2O → 2 R3SnOSnR'3
The Sn-O-Sn bonds in simple organic derivatives are reactive toward carboxylic acid esters to give unsymmetrical distannoxanes:
2 R2SnO + R'CO2R" → R"OSnR2-O-SnR2O2CR'
References
- Davies, Alwyn George. (2004) Organotin Chemistry, 2nd Edition Weinheim: Wiley-VCH. ISBN 978-3-527-31023-4