Sporolides
Sporolides A and B are novel polycyclic macrolides from the obligate marine bacterium Salinispora tropica (which is found in ocean sediment) that are composed of a chlorinated cyclopenta[a]indene ring and a cyclohexenone moiety.[1] They are only the second class of compounds isolated from Salinispora, and their unique carbon skeleton provides a clear indication of the tremendous potential of marine actinomycetes as a source of novel secondary metabolites.[2] The structures and absolute stereochemistries of both metabolites were elucidated using a combination of NMR spectroscopy and X-ray crystallography, and are considered unique for two reasons:
 | |
| Identifiers | |
|---|---|
| ChemSpider |
|
| Chemical and physical data | |
| Formula | C24H23ClO12 |
| Molar mass | 538.89 g·mol−1 |
| | |
- both compounds appear to be polyketides and therefore derived from acetate units,
- the number of oxidized carbons is amazing, with 21 of 24 carbons either oxygenated or sp2 - hybridized.
The complex aromatic structure of the sporolides is hypothesized to be derived from an unstable nine-membered ring enediyne precursor, which undergoes Bergman cyclization to generate a p-benzyne intermediate. A nucleophilic attack by chloride accounts for the 1:1 mixtures and for the single chlorine in enediyne-derived natural products. This mechanism has been recently demonstrated in laboratory experiments,[3] and can account for the biosynthesis of sporolides.

Biosynthesis
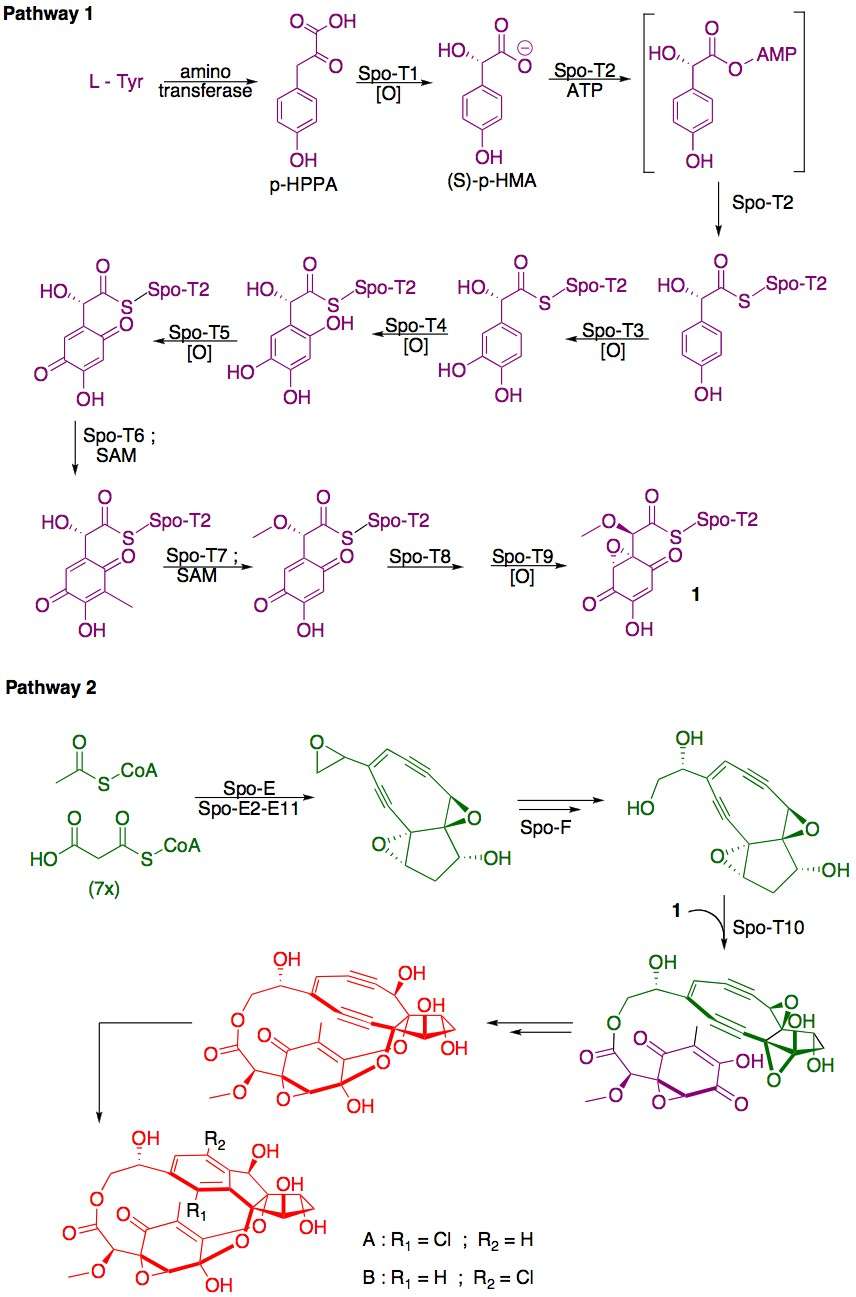
Total Synthesis
First stereoselective synthesis was reported by K. C. Nicolaou, more specifically, Sporolide B, through a highly stereoselective and convergent strategy that involves two important cycloaddition reactions: a thermally induced intramolecular [4+2] cycloaddition reaction involving an o-quinone and a tetrasubstituted olefin to form the macrocyclic structure of the molecule, and a ruthenium-catalyzed, intermolecular [2+2+2] cycloaddition reaction between two acetylenic units.[4]

References
- McGlinchey, R. P.; Nett, M.; Moore, B. J. Am. Chem. Soc. 2008, 130, 2406-2407.
- Buchanan, G. O.; Williams, P. G.; Feling, R. H.; Kauffman, C. A.; Jensen, P. R.; Fenical, W. Org. Lett. 2005, 7, pp. 2731-2734.
- Perrin, C. L.; Rodgers, B. L.; O' Connor, J. M.; J. Am. Chem. Soc. 2007, 129, (15), 4795-4799.
- Nicolaou, K. C.; Tang, Yefeng; Wang, Jianhua. Angew. Chem. Int. Ed. 2009, 48 (19), 3449-34.