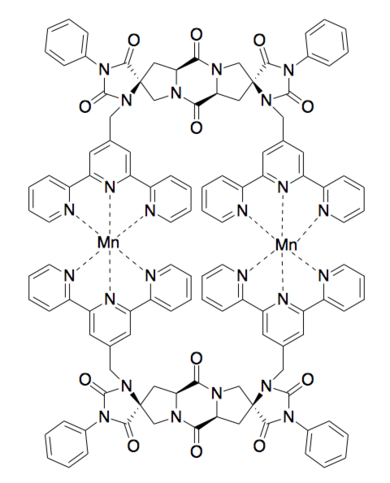
Spiroligomer
Spiroligomers (also known as bis-peptides) are synthetic oligomers made by coupling pairs of bis-amino acids into a fused ring system.[1] Spiroligomers are rich in stereochemistry and functionality because of the variety of bis-amino acids that are capable of being incorporated during synthesis.[2] Due to the rigidity of the fused ring system,[3] the three-dimensional shape of a spiroligomer – as well as the display of any functional groups – can be predicted, allowing for molecular modeling and dynamics.
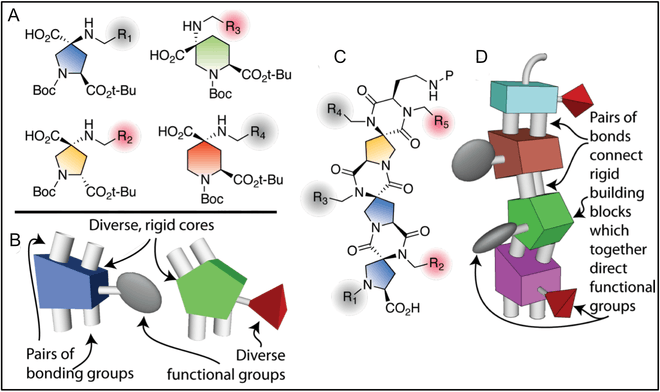
Synthesis
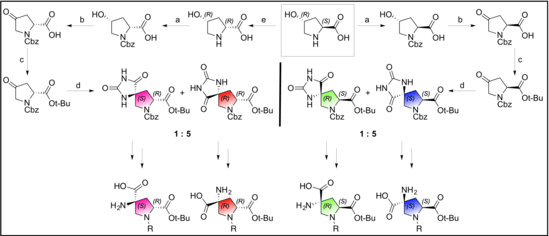
Spiroligomers are synthesized in a step-wise approach by adding a single bis-amino acid at each stage of the synthesis. This stepwise elongation allows for complete control of the stereochemistry, as any bis-amino acid can be incorporated to allow for elongation; or any mono-amino acid can be added to terminate a chain. This can be accomplished using either solution-phase or solid-phase reactions.[4] The original synthesis of spiroligomers allowed for functionalization on the ends of the oligomers, but it did not allow for the incorporation of functionality on the interior diketopiperazine (DKP) nitrogens.[5] Much work has been done to allow for the functionalization of the entire Spiroligomer, as opposed to just the ends.[2] By exploiting a neighboring group effect,[6] spiroligomers can be synthesized with a variety of functional groups along the length of the molecule.
Structure
Spiroligomers can be synthesized in any direction, and between any pair of bis-amino acids. Spiroligomer diketopiperazines can be created between either end of a bis-amino acid. Spiroligomers are known to be conformationally rigid, due to the fused-ring backbone.[3]
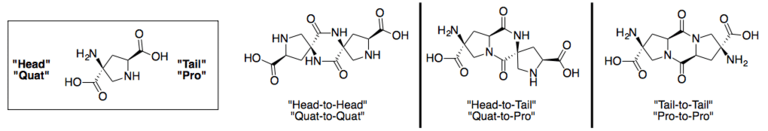
Chemical characteristics
Spiroligomers are peptidomimetics, completely resistant to proteases, and not likely to raise an immune response.
Uses
Spiroligomers have been utilized for a variety of applications which include catalysis, protein binding, metal-binding, molecular scaffolds, and charge-transfer studies, et al.
Catalysis
Two unique types of spiroligomer catalysts (spiroligozymes) have been developed, an esterase mimic and a Claisen catalyst.
Transesterification
The first spiroligomer catalyst was an esterase-mimic, which catalyzed the transfer of a trifluoroacetate group.[7]
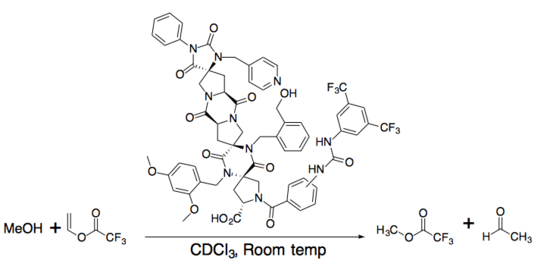
Aromatic Claisen rearrangement
The second spiroligomer catalyst accelerated an aromatic Claisen rearrangement with a catalytic dyad similar to that found in ketosteroid isomerase.[8]
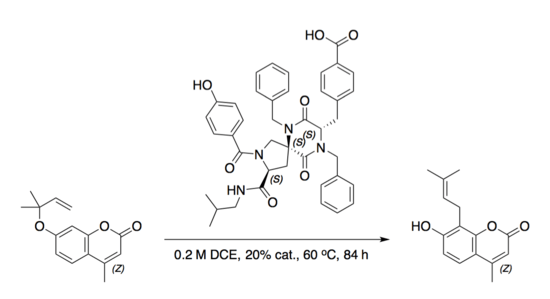
Protein binding
A spiroligomer was designed to mimic P53 and bind HDM2. The molecule enters cells through passive diffusion, and this mimic was shown to stabilize HDM2 in cell culture.[9]
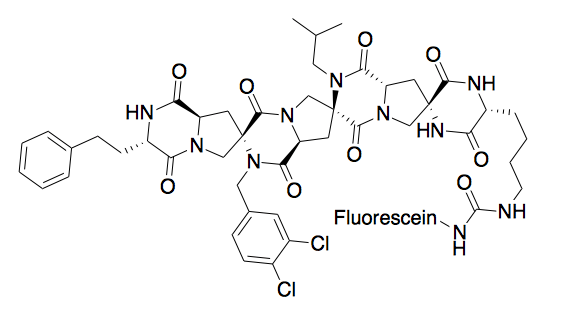
Molecular scaffolds
Rods used for distance measuring with spin probes.[3]
Electron transfer
Donor-Bridge-Acceptor[11]
Other uses
Possible applications that are currently investigated include the binding and inactivation of cholera toxin and the cross linkage of surface proteins of various viruses (HIV, Ebola virus). Further the group of Christian Schafmeister developed molecular hinges, which can be used for the construction of molecular machines, such as nano-valves or data storage systems.[12]
References
- Christian E. Schafmeister, Zachary Z. Brown, and Sharad Gupta "Shape-Programmable Macromolecules" Accounts of Chemical Research, (2008), 41(10), 1387-1398 doi:10.1021/ar700283y
- Zachary Z. Brown and Christian E. Schafmeister, "Synthesis of Hexa- and Pentasubstituted Diketopiperazines from Sterically Hindered Amino Acids" Organic Letters, (2010), 12(7), 1436-1439 doi:10.1021/ol100048g
- Gregory H. Bird, Soraya Pornsuwan, Sunil Saxena, and Christian E. Schafmeister, "Distance Distributions of End-Labeled Curved Bispeptide Oligomers by Electron Spin Resonanace" ACSNano (2008), 2(9), 1857-1864 doi:10.1021/nn800327g
- Zachary Z. Brown, Jennifer Alleva, and Christian E. Schafmeister, "Solid-Phase Synthesis of Functionalized Bis-Peptides" Biopolymers, (2011), 96(5), 578-585 doi:10.1002/bip.21591
- Christopher G. Levins and Christian E. Schafmeister, "The Synthesis of Functionalized Nanoscale Molecular Rods of Defined Length" Journal of the American Chemical Society, (2003), 125(16), 4702-4703 doi:10.1021/ja0293958
- Zachary Z. Brown and Christian E. Schafmeister, "Exploiting an Inherent Neighboring Group Effect of alpha-Amino Acids to Synthesize Extremely Hindered Dipeptides" Journal of the American Chemical Society, (2008), 130(44), 14382-14383 doi:10.1021/ja806063k
- Mahboubeh Kheirabadi and Christian Schafmeister, et al, "Spiroligozymes for Transesterifications: Design and Relationship of Structure to Activity" Journal of the American Chemical Society, (2012), 134, 18345-18353 doi:10.1021/ja3069648
- Matthew F. L. Parker and Christian E. Schafmesiter, et al, "Acceleration of an Aromatic Claisen Rearrangement via a Designed Spiroligozyme Catalyst that Mimics the Ketosteroid Isomerase Catalytic Dyad" Journal of the American Chemical Society, (2014), 136(10), 3817-3827 doi:10.1021/ja409214c
- Zachary Z. Brown, Kavitha Akula, and Christian Schafmeister, et al, "A Spiroligomer alpha-Helix Mimic That Binds HDM2, Penetrates Human Cells, and Stabilizes HDM2 in Cell Culture" PLOSOne (2012), 7(10), doi:10.1371/journal.pone.0045948
- Shivaiah Vaddypally, Chongsong Xu, Senzhi Zhao, Yanfeng Fan, Christian E. Schafmeister and Michael J. Zdilla "Architectural Spiroligomers Designed for Binuclear Metal Complex Templating" Inorganic Chemistry, (2013), 52, 6457-6463 doi:10.1021/ic4003498
- Subhasis Chakrabarti, Matthew F. L. Parker, Christopher W. Morgan, Christian E. Schafmeister, and David H. Waldeck, "Experimental Evidence for Water Mediated Electron Transfer Through Bis-Amino Acid Donor-Bridge-Acceptor Oligomers" Journal of the American Chemical Society, (2009), 131(6), 2044-2045 doi:10.1021/ja8079324
- Levins CG, Schafmeister CE. The synthesis of curved and linear structures from a minimal set of monomers. Journal of Organic Chemistry, 70, p. 9002, 2005. doi:10.1002/chin.200605222