Sperm oil
Sperm oil is a waxy liquid obtained from sperm whales. It is a clear, yellowish liquid with a very faint odor. Sperm oil has a different composition from common whale oil, obtained from rendered blubber. Although it is traditionally called an "oil", it is technically a liquid wax. It is composed of wax esters with a small proportion of triglycerides, an ester of an unsaturated fatty acid and a branched-chain fatty alcohol.[1] It is a natural antioxidant and heat-transfer agent.[1] In the late-18th and early-19th centuries, sperm oil was prized as an illuminant for its bright, odorless flame and as a lubricant for its low viscosity and stability. It was supplanted in the late 19th century by less expensive alternatives such as kerosene and petroleum-based lubricants. With the 1987 international ban on whaling, sperm oil is no longer legally sold.[2]

The oil from bottlenose whales was sometimes called "Arctic sperm oil". It was cheaper and inferior to true sperm oil.[3][4]
Processing
.svg.png)
After killing a sperm whale, the whalers would pull the carcass alongside the main whaleship with the capture boat, or the whaleship would sail to the floating carcass if the weather conditions were favorable. The whale was then fastened to the starboard (right) side of the ship with heavy chains, whereby the crew then proceeded to erect a “cutting stage”, which primarily consisted of a wooden plank platform above the carcass.[5]
It was important to process the whale as soon as possible to prevent sharks and other predators from feasting on too much of the valuable carcass. The whaling crew divided into two watches, worked six-hour shifts, day and night, until the job was finished. The process could take from several hours to several days, depending on the size and species of the whale, the skill of the crew, and the weather.[5]
Blubber was stripped from the entire length of the whale (with other parts of the carcass often harvested for fashion products such as in the case of the Baleen whale), but the primary source of sperm oil was the spermaceti organ and the junk (or "melon"), the organs that serve to focus and modulate the animal's vocalizations.[5] A sperm whale's spermaceti organ may contain as much as 1,900 litres of substance.[6] The matter from these organs was stored in casks to be processed on land; sometimes it was boiled first to prevent it going rancid. The blubber also contained smaller proportions of spermaceti, which was obtained by boiling the blubber on the ship itself. On land, the casks of head-matter were allowed to chill during the winter, causing it to congeal into a spongy and viscous mass. The congealed matter was then loaded into wool sacks and placed in a press to squeeze out its liquid. This liquid was bottled and sold as "winter-strained sperm oil". This was the most valuable product: an oil that remained liquid in freezing winter temperatures. When spring came and the leftover solid matter melted a bit, the liquid was strained off and sold as "spring-strained sperm oil". In summer, the matter melted some more and the liquid was strained off to leave a fully solid wax. This wax, brown in color, was then bleached and sold as "spermaceti wax".[7][8]
Chemistry
| specific gravity | 0.884 at 15.6 °C[9] |
| flash point | 260-266 °C[10] |
| saponification value | 120-150.3[11] |
| unsaponifiable matter | 17.5-44.0%[11] |
| refractive index | 1.4649 at 15.6 °C[12][13] |
| iodine number (Wijs) | 70.4-96.4[11] |
| viscosity | 21-23 cSt at 37.5 °C[14] |
| viscosity index | 180[15] |
Sperm oil has a fairly low viscosity (roughly equal to coconut oil).[14] It retains its viscosity in high temperatures better than most oils. It does not tend to become rancid, dry out, or corrode metals. Sperm oil cannot be easily hydrogenated, and thus could not be used to make soap or margarine.[16][17] It is fairly resistant to oxidization.[18]
Spermaceti is a liquid wax, composed mostly of wax esters (chiefly cetyl palmitate) and a smaller proportion of triglycerides,[19] with oleic acid being the most common fatty acid. The proportion of wax esters in the spermaceti organ increases with the age of the whale: 38-51% in calves, 58-87% in adult females, and 71-94% in adult males. The blubber oil of the whale is about 66% wax.[20] When cooled to below 30 °C, the waxes in spermaceti begin to crystallize.[21]
Winter-strained sperm oil is roughly two-thirds wax esters and one third triglycerides.[22][23] Most of the carbon chains are unsaturated, with 18:1 being the most common.[24] Unlike other toothed whales save the Amazon river dolphin, most of the carbon chains in the wax esters are relatively long (C10-C22).[20]
Applications
Sperm oil was particularly prized as an illuminant in oil lamps, as it burned more brightly and cleanly than any other available oil and gave off no foul odor.[8] It was replaced in the late-19th century by cheaper, more efficient kerosene.[25]
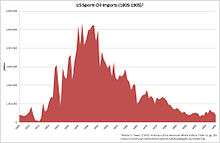
In the US, whale oil was used in cars as an additive to automatic transmission fluid until it was banned by the Endangered Species Act.[26] Prior to 1972, over 30 million lb (14 million kg) of sperm whale oil was used annually in lubricants because of its exceptional lubricity and heat stability.[27] In 1972, the sperm whale was listed as an Endangered Species. The following year, the US Congress amended the Endangered Species Act, outlawing the killing of whales and the use of their oil.[27] The loss of whale oil had a profound impact in the automotive industry,[28] where for example, transmission failures rose from under 1 million in 1972 to over 8 million by 1975.[27]
Sperm oil was a popular lubricant. It worked well for fine, light machinery such as sewing machines and watches because it is thin, does not congeal or dry out and does not corrode metals. It was also used in heavy machinery such as locomotives and steam-powered looms because it can withstand high temperatures.[29] In the late 20th century, Jojoba oil was discovered to be a better substitute for high-friction applications because it is even more stable at high temperatures. This caused sperm oil's price to collapse to a tenth of its previous value.[30]
Because of its very low freezing point, sperm oil saw widespread use in the aerospace industry.[31]
Sperm oil was used to protect metals from rust. A coat of sperm oil provided a temporary protection for the metal components in firearms, because it did not dry out or gum up.[32][33] It was the basis of the original (but not current) Rust-Oleum.
Gallery
 A spermaceti press
A spermaceti press Strained sperm oil being bottled
Strained sperm oil being bottled A spermaceti wax candle
A spermaceti wax candle
.jpg) Sperm whales celebrate the discovery of new oil wells in Pennsylvania. The proliferation of mineral oils reduced the demand for their species' oil. (1861 cartoon from Vanity Fair)
Sperm whales celebrate the discovery of new oil wells in Pennsylvania. The proliferation of mineral oils reduced the demand for their species' oil. (1861 cartoon from Vanity Fair)
References
- [Transmission Digest, Volume 26, No. 2, October 2006, "The Science of Synthetic Sperm Whale Oil"]
- http://www.publicaffairs.noaa.gov/releases2004/jun04/noaa04-r150.html
- Julius Lewkowitsch (1904). Chemical technology and analysis of oils, fats, and waxes. pg 870
- "Archived copy". Archived from the original on 2013-11-22. Retrieved 2014-03-13.CS1 maint: archived copy as title (link)
- Museum, New Bedford Whaling. "Whales and Hunting". New Bedford Whaling Museum. Retrieved 2020-01-06.
- https://ntrs.nasa.gov/archive/nasa/casi.ntrs.nasa.gov/19720017437.pdf
- ""Beginning with Candle Making A History of the Whaling Museum " Historic Nantucket article from the Nantucket Historical Association". www.nha.org.
- Wilson Heflin (2004). Herman Melville's Whaling Years. pg 232
- Emil F. Dieterichs (1916). A Practical Treatise On Friction, Lubrication, Fats And Oils. pg 22
- Richard P. Pohanish (2005). HazMat Data: For First Response, Transportation, Storage, and Security. pg 865
- Moninder Mohan Chakrabarty (2009). Chemistry And Technology Of Oils And Fats. pg 183
- Abraham G. Blakeley , Edmund A. Reilly (1917). Some Data on Sperm Oils Used for Burning Purposes. The Journal of Industrial and Engineering Chemistry, Dec 1917. pg 1099
- J. N. Goldsmith (1921). Table of Refractive Indices. pg 239
- "Liquids - Kinematic Viscosities". www.engineeringtoolbox.com.
- William Gordon Forbes (1943). Lubricants and cutting oils for machine tools. pg 69
- Joh. N. Tønnessen, Arne Odd Johnsen (1984). The History of Modern Whaling, pg 228.
- Robert Lloyd Webb (1988). On the Northwest: Commercial Whaling in the Pacific Northwest, 1790-1967. pg 144
- Czesław Kajdas, S. S. K. Harvey, E. Wilusz (1990). Encyclopedia of Tribology, Volume 15. pg 308.
- "Archived copy" (PDF). Archived from the original (PDF) on 2013-06-01. Retrieved 2012-10-29.CS1 maint: archived copy as title (link)
- William F. Perrin, Bernd Würsig, J. G. M. Thewissen (2002). Encyclopedia of Marine Mammals. pg 1164
- Malcolm R. Clarke (1978). Physical Properties of Spermaceti Oil in the Sperm Whale. Journal of the Marine Biological Association of the United Kingdom Archived 2008-12-17 at the Wayback Machine
- G. F. Spencer, W. H. Tallent (1973). Sperm whale oil analysis by gas chromatography and mass spectrometry. Journal of the American Oil Chemists' Society
- J.W. Hagemann and J.A. Rothfus (1978). Oxidative Stability of Wax Esters by Thermogravimetric Analysis. JOURNAL OF THE AMERICAN OIL CHEMISTS' SOCIETY, Vol .56, No.6. Pages: 629-631
- Gayland F. Spencer (1978). Alkoxy-Acyl Combinations in the Wax Esters from Winterized Sperm Whale Oil by Gas Chromatography-Mass Spectrometry
- "Thefreemanonline.org". www.thefreemanonline.org.
- Information, Reed Business (1 May 1975). "New Scientist". Reed Business Information – via Google Books.
- Landis, Phil. Gears for the Transmission Rebuilding Industry. Oxnard: Automatic Transmission Rebuilders Association, 2010. Print.
- "Transmission Problems in Cars Linked to Ban on Whale Killing", New York Times, April 17, 1975. Retrieved 1 March 2020.
-
- Paul Lucier, Scientists and Swindlers (2008). Consulting on Coal and Oil in America. pg 152.
- James R. McGuigan, R. Charles Moyer, Frederick H. deB Harris (2010). Managerial Economics, pg 29.
- American Whaling, New Bedford Whaling Museum. Modified August 22, 2014.
- Roy F. Dunlap (1963). Gunsmithing: The complete sourcebook of firearms design, construction, alteration, and restoration for amateur and professional gunsmiths. pg= 84.
- William S. Brophy (1985). The Springfield 1903 Rifles. pg 71.