Solketal
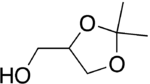
Solketal is a protected form of glycerol with an isopropylidene acetal group joining two neighboring hydroxyl groups. Solketal contains a chiral center on the center carbon of the glycerol backbone, and so can be purchased as either the racemate or as one of the two enantiomers. Solketal has been used extensively in the synthesis of mono-, di- and triglycerides by ester bond formation. The free hydroxyl groups of solketal can be esterified with a carboxylic acid to form the protected monoglyceride, where the isopropylene group can then be removed using an acid catalyst in aqueous or alcoholic medium. The unprotected diol can then be esterified further to form either the di- or triglyceride.
 | |
| Names | |
|---|---|
| IUPAC name
(2,2-Dimethyl-1,3-dioxolan-4-yl)methanol | |
| Other names
Isopropylidene glycerol | |
| Identifiers | |
| |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.002.626 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C6H12O3 | |
| Molar mass | 132.159 g·mol−1 |
| Appearance | clear colorless liquid |
| Density | 1.063 g/mL at 25 °C |
| Boiling point | 188 to 189 °C (370 to 372 °F; 461 to 462 K) |
| Miscible | |
| Solubility | Miscible in most organic solvents (alcohols, ethers, hydrocarbons) |
| Hazards | |
| Flash point | 80 °C (176 °F; 353 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
References
- Mary Renoll and Melvin S. Newman (1955). "dl-ISOPROPYLIDENEGLYCEROL". Organic Syntheses. 28: 73.; Collective Volume, 3, p. 502
- Sanderson, John R.; Lin, Jiang J.; Duranleau, Roger G.; Yeakey, Ernest L.; Marquis, Edward T. (1988). "Free radicals in organic synthesis. A novel synthesis of glycerol based on ethylene glycol and formaldehyde". Journal of Organic Chemistry. 53 (12): 2859. doi:10.1021/jo00247a043.
- "Solketal". Logo of chemBlink Inc. Online Database of Chemicals from Around the World. Archived from the original on 31 October 2010.
- Matsumoto, Yoshihiko; Mita, Keisuke; Hashimoto, Keiji; Iio, Hideo; Tokoroyama, Takashi (1996). "Selective cleavage of ethers using silica-alumina gel catalysts prepared by the sol-gel method". Tetrahedron. 52 (28): 9387. doi:10.1016/0040-4020(96)00501-7.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.