Sodium tert-butoxide
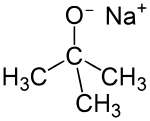
Sodium tert-butoxide is the chemical compound with the formula (CH3)3CONa.[2] It is a strong base and a non-nucleophilic base. It is flammable and moisture sensitive. It is sometimes written in chemical literature as sodium t-butoxide. It is similar in reactivity to the more common potassium tert-butoxide.
 | |
| Names | |
|---|---|
| IUPAC name
Sodium 2-methylpropan-2-olate | |
Other names
| |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.011.584 |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C4H9NaO | |
| Molar mass | 96.105 g·mol−1 |
| Density | 1.025 g/cm3 |
| Acidity (pKa) | 19[1] |
| Hazards | |
| Safety data sheet | |
| Flash point | 14 °C (57 °F; 287 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
The compound can be produced by treating tert-butyl alcohol with sodium hydride.[3]
Uses
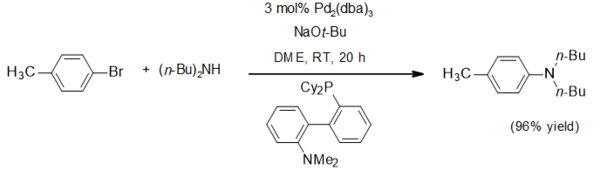
The main application for sodium tert-butoxide is as a non-nucleophilic base. It has been widely used in the Buchwald–Hartwig amination, as in this typical example[4]:
Structure
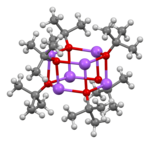
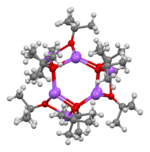
Sodium tert-butoxide forms clusters in the solid state, both hexamers[5] and nonamers.[6]
 |  |
Toxicity
Sodium t-butoxide is extremely destructive to tissue of the mucous membranes and upper respiratory tract, eyes, and skin.
References
- Dewick, Paul M. (2013-03-20). Essentials of Organic Chemistry: For Students of Pharmacy, Medicinal Chemistry and Biological Chemistry. ISBN 9781118681961.
- http://www.sigmaaldrich.com/catalog/ProductDetail.do?lang=en&N4=359270%7CALDRICH&N5=SEARCH_CONCAT_PNO%7CBRAND_KEY&F=SPEC}
- PM. Dewick, 2013. Essentials of Organic Chemistry: For Students of Pharmacy, Medicinal Chemistry and Biological Chemistry. John Wiley & Sons; p. 157. ISBN 978-1-118-68196-1
- Yang, Bryant H.; Buchwald, Stephen L. (1999). "Palladium-catalyzed amination of aryl halides and sulfonates". Journal of Organometallic Chemistry. 576 (1–2): 125–146. doi:10.1016/S0022-328X(98)01054-7.
- E. Østreng, H. H. Sønsteby, S. Øien, O. Nilsen, H. Fjellvåg (2014). "Atomic layer deposition of sodium and potassium oxides: evaluation of precursors and deposition of thin films". Dalton Trans. 43: 16666–16672. doi:10.1039/C4DT01930J.CS1 maint: uses authors parameter (link)
- H. Nekola, F. Olbrich, U. Behrens (2002). "Kristall‐ und Molekülstrukturen von Lithium‐ und Natrium‐tert‐butoxid". Z. Anorg. Allg. Chem. 628 (9–10): 2067–2070. doi:10.1002/1521-3749(200209)628:9/10<2067::AID-ZAAC2067>3.0.CO;2-N.CS1 maint: uses authors parameter (link)