Sodium polyaspartate
Sodium polyaspartate is a sodium salt of polyaspartic acid. It is biodegradable condensation polymer based on the amino acid aspartic acid.
 | |
| Names | |
|---|---|
| IUPAC name
polyaspartic acid sodium salt | |
| Identifiers | |
| |
CompTox Dashboard (EPA) |
|
| Properties | |
| (C4H4NNaO3)n | |
| Molar mass | variable |
| Hazards | |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Polymerization
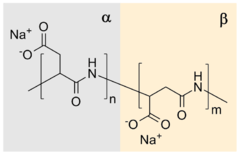
The polymerization reaction is an example of a step-growth polymerization to a polyamide and in one practical procedure[1] aspartic acid is simply heated to 180 °C resulting in water release and the formation of a poly(succinimide). In the subsequent step, this polymer is reacted with sodium hydroxide in water, which hydrolyzes one of the two amide bonds of the succinimide ring to form a sodium carboxylate. The remaining amide bond is thus the linkage between successive aspartate units. Each aspartate unit is identified as α or β according to which carbonyl of it is part of the polymer chain. The α form has one carbon in the backbone in addition to the carbonyl itself (and a two-carbon sidechain) whereas the β form has two carbons in the backbone in addition to the carbonyl itself (and a one-carbon sidechain). This reaction gives a sodium poly(aspartate) copolymer composed of approximately 30% α-linkages and 70% β-linkages.
.svg.png)
Uses
This material can be synthesized in an environmentally friendly way and is biodegradeable, thus it is a green alternative to several materials such as sodium polyacrylate used in disposable diapers and agriculture.[2][3]
In addition and due to its water-solubility and ability to chelate metal ions, polyaspartate is used as a biodegradeable anti-scaling agent and a corrosion inhibitor.[4][5]
References
- Bennett GD (2005). "A Green Polymerization of Aspartic Acid for the Undergraduate Organic Laboratory". J. Chem. Educ. 82 (9): 1380–1381. Bibcode:2005JChEd..82.1380B. doi:10.1021/ed082p1380.
- Gross, R. A.; Kalra, B. (2002). "Biodegradable Polymers for the Environment". Science. 297 (5582): 803–807. Bibcode:2002Sci...297..803G. doi:10.1126/science.297.5582.803. PMID 12161646.
- "Presidential Green Chemistry Challenge Awards: 1996 Small Business Award: Donlar Corporation (now NanoChem Solutions, Inc.): Production and Use of Thermal Polyaspartic Acid". US Environmental Protection Agency.
- Low, K. C.; Wheeler, A. P.; Koskan, L. P. (1996). Commercial poly(aspartic acid) and Its Uses. Advances in Chemistry Series. 248. Washington, D.C.: American Chemical Society.
- Thombre, S.M.; Sarwade, B.D. (2005). "Synthesis and Biodegradability of Polyaspartic Acid: A Critical Review" (PDF). Journal of Macromolecular Science, Part A. 42 (9): 1299–1315. doi:10.1080/10601320500189604.
