Sodium naphthalenide
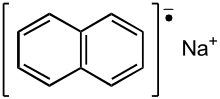
Sodium naphthalenide, also known as sodium naphthalide, is an organic salt with the chemical formula Na+C10H8−. In the research laboratory, it is used as a reductant in the synthesis of organic, organometallic, and inorganic chemistry. It has not been isolated as a solid, but it is usually prepared fresh before use.[1]
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Sodium naphthalenide | |
| Systematic IUPAC name
Sodium naphthalen-1-ide | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.020.420 |
| EC Number |
|
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C10H8Na | |
| Molar mass | 151.164 g·mol−1 |
| Related compounds | |
Other anions |
Sodium cyclopentadienide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Preparation and properties
The alkali metal naphthalenides are prepared by stirring the metal with naphthalene in an ethereal solvent, usually as tetrahydrofuran or dimethoxyethane. The resulting salt is dark green.[2][3][4] The anion is a radical, giving a strong EPR signal near g = 2.0, with a reduction potential near -2.5 V vs NHE. Its deep green color arises from absorptions centered at 463, 735 nm.[1]
The anion is strongly basic, and a typical degradation pathway involves reaction with water and related protic sources. These reactions afford dihydronaphthalene:
- 2 NaC10H8 + 2 H2O → C10H10 + C10H8 + 2 NaOH
Related reagents
For some synthetic operations, sodium naphthalenide is excessively reducing (too negative) or too insoluble. In such cases, alternative reductants are selected.
- Sodium acenaphthalenide is milder by about 0.75 V, reflecting the milder reduction potential of polycyclic aromatic compounds.
- Lithium biphenyl is a THF-soluble species related to lithium naphthalenide except that it is a poorer ligand.[5]
- Sodium 1-methylnaphthalenide is more soluble than sodium naphthalenide, which is useful for low-temperature reductions.[6]

References
- N. G. Connelly and W. E. Geiger, "Chemical Redox Agents for Organometallic Chemistry", Chem. Rev. 1996, 96, 877-910. doi:10.1021/cr940053x
- Corey, E. J.; Gross, Andrew W. (1993). "tert-Butyl-tert-octylamine". Organic Syntheses.; Collective Volume, 8, p. 93
- Cotton, F. Albert; Wilkinson, Geoffrey (1988), Advanced Inorganic Chemistry (5th ed.), New York: Wiley-Interscience, p. 139, ISBN 0-471-84997-9
- Greenwood, Norman N.; Earnshaw, Alan (1984). Chemistry of the Elements. Oxford: Pergamon Press. p. 111. ISBN 978-0-08-022057-4.
- Rieke, Reuben D.; Wu, Tse-Chong & Rieke, Loretta I. (1995). "Highly Reactive Calcium for the Preparation of Organocalcium Reagents: 1-Adamantyl Calcium Halides and Their Addition to Ketones: 1-(1-Adamantyl)cyclohexanol". Org. Synth. 72: 147. doi:10.15227/orgsyn.072.0147.
- Liu, X.; Ellis, J. E. (2004). "Hexacarbonylvanadate(1−) and Hexacarbonylvanadium(0)". Inorg. Synth. 34: 96–103. doi:10.1002/0471653683.ch3. ISBN 0-471-64750-0.