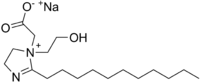
Sodium lauroamphoacetate
Sodium lauroamphoacetate is zwitterionic surfactant of the amphoacetate class. It is used as a very mild cleaning agent originally used in shampoos and body washes for infants but it now sees broader use in other personal care products.[1]
 | |
| Names | |
|---|---|
| IUPAC name
Sodium 2-[1-(2-hydroxyethyl)-2-undecyl-4,5-dihydroimidazol-1-ium-1-yl]acetate | |
| Other names
Sodium lauroamphocetate; Lauroamphoacetate; Sodium lauramphoacetate; Sodium lauromphoacetate; Sodium lauraamphoacetate; Sodium lauroamphoacetate; Lauroamphoglycinate; Lauroamphoglycinate; Sodium lauroamphoacetate | |
| Identifiers | |
3D model (JSmol) |
|
CompTox Dashboard (EPA) |
|
| |
| Properties | |
| C18H34N2NaO3 | |
| Molar mass | 349.471 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Synthesis
Sodium lauroamphoacetate is produced in a 2 step process. Firstly lauric acid reacts with aminoethylethanolamine (AEEA); this initially produces the amide however heating causes this to cyclize to give the imidazoline group. This reacts with 1 equivalent of sodium chloroacetate to give the final product. A reaction with 2 equivalents gives the di-acetate, which is also marketed as di-sodium lauroamphoacetate.[1]
Safety
Sodium lauroamphoacetate is exceedingly mild,[2] and cases of skin irritation are rare but not unheard of. It has been proposed that these instances are caused not by sodium lauroamphoacetate itself but rather by failures in quality control which result in it being contaminated with AEEA.[3]
References
- Farn, Richard J. (2006). Chemistry and technology of surfactants. Oxford: Blackwell Pub. pp. 173–174. ISBN 978-14051-2696-0.
- Mehling, A.; Kleber, M.; Hensen, H. (May 2007). "Comparative studies on the ocular and dermal irritation potential of surfactants". Food and Chemical Toxicology. 45 (5): 747–758. doi:10.1016/j.fct.2006.10.024.
- Foti, Caterina; Bonamonte, Domenico; Mascolo, Giuseppe; Tiravanti, Giovanni; Rigano, Luigi; Angelini, Gianni (September 2001). "Aminoethylethanolamine: a new allergen in cosmetics?". Contact Dermatitis. 45 (3): 129–133. doi:10.1034/j.1600-0536.2001.045003129.x.