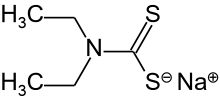
Sodium diethyldithiocarbamate
Sodium diethyldithiocarbamate is the organosulfur compound with the formula NaS2CN(C2H5)2.
 | |
 | |
.jpg) | |
| Names | |
|---|---|
| IUPAC name
sodium (diethylcarbamothioyl)sulfanide | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.005.192 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C5H10NS2Na | |
| Molar mass | 171.259 g/mol (anhydrous) |
| Appearance | White, slightly brown, or slightly pink crystalline solid |
| Density | 1.1 g/cm3 |
| Melting point | 95 °C (203 °F; 368 K) |
| Soluble | |
| Solubility | soluble in alcohol, acetone insoluble in ether, benzene |
| Hazards | |
| Main hazards | Harmful |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Preparation
This salt is obtained by treating carbon disulfide with diethylamine in the presence of sodium hydroxide:
- CS2 + HN(C2H5)2 + NaOH → NaS2CN(C2H5)2 + H2O
Other dithiocarbamates can be prepared similarly from secondary amines and carbon disulfide. They are used as chelating agents for transition metal ions and as precursors to herbicides and vulcanization reagents.
Oxidation to thiuram disulfide
Oxidation of sodium diethyldithiocarbamate gives the disulfide, also called a thiuram disulfide (Et = ethyl):
- 2 NaS2CNEt2 + I2 → Et2NC(S)S-SC(S)NEt2 + 2 NaI
This disulfide is marketed as an anti-alcoholism drug under the labels Antabuse and Disulfiram. Chlorination of the above-mentioned thiuram disulfide affords the thiocarbamoyl chloride.[1]
Ligand bonding
The diethyldithiocarbamate ion chelates to many "softer" metals via the two sulfur atoms. Other more complicated bonding modes are known including binding as unidentate ligand and a bridging ligand using one or both sulfur atoms.[2]
Spin trapping of nitric oxide radicals
Complexes of dithiocarbamates with iron provide one of the very few methods to study the formation of nitric oxide (NO) radicals in biological materials. Although the lifetime of NO in tissues is too short to allow detection of this radical itself, NO readily binds to iron-dithiocarbamate complexes. The resulting mono-nitrosyl-iron complex (MNIC) is stable, and may be detected with Electron Paramagnetic Resonance (EPR) spectroscopy.[3][4][5]
In cancer
The zinc chelation of diethyldithiocarbamate inhibits metalloproteinases, which in turn prevents the degradation of extracellular matrix, an initial step in cancer metastasis and angiogenesis.[6]
Antioxidant
Diethyldithiocarbamate inhibits superoxide dismutase, which can both have antioxidant and oxidant effects on cells, depending on the time of administration.[6]
References
- Goshorn, R. H.; Levis, Jr., W. W. ;Jaul, E.; Ritter, E. J. (1963). "Diethylthiocarbamyl Chloride". Organic Syntheses.CS1 maint: multiple names: authors list (link); Collective Volume, 4, p. 307
- Cotton, F. Albert; Wilkinson, Geoffrey; Murillo, Carlos A.; Bochmann, Manfred (1999), Advanced Inorganic Chemistry (6th ed.), New York: Wiley-Interscience, ISBN 0-471-19957-5
- Henry Y.; Guissani A.; Ducastel B. (eds); "Nitric oxide research from chemistry to biology: EPR spectroscopy of nitrosylated compounds." Landes, Austin 1997.
- Vanin, A.F.; Huisman, A.; van Faassen, E.E. (2002). "Iron dithiocarbamates as spin trap for nitric oxide: Pitfalls and successes". Methods in Enzymology. 359: 27–42. doi:10.1016/s0076-6879(02)59169-2. PMID 12481557.
- van Faassen E.E.; Vanin A.F. (eds); "Radicals for life: The various forms of nitric oxide." Elsevier, Amsterdam 2007.
- diethyldithiocarbamate National Cancer Institute - Drug Dictionary
Further reading
- Cvek B, Dvorak Z (2007). "Targeting of nuclear factor-kappaB and proteasome by dithiocarbamate complexes with metals". Curr. Pharm. Des. 13 (30): 3155–67. doi:10.2174/138161207782110390. PMID 17979756.