Sodium calcium edetate
Sodium calcium edetate (sodium calcium EDTA), also known as edetate calcium disodium among other names, is a medication primarily used to treat lead poisoning,[1] including both short-term and long-term lead poisoning.[2] Sodium calcium edetate came into medical use in the United States in 1953.[2]
 | |
| Clinical data | |
|---|---|
| Trade names | Calcium disodium versenate, others |
| Other names | edetate calcium disodium, sodium calcium edetate |
| AHFS/Drugs.com | Monograph |
| Pregnancy category |
|
| Routes of administration | IV, IM |
| Drug class | chelating agent |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| E number | E385 (antioxidants, ...) |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.482 |
| Chemical and physical data | |
| Formula | C10H12CaN2Na2O8 |
| Molar mass | 374.270 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Chelation agent
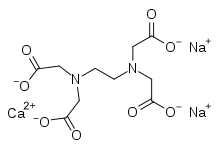
Sodium calcium edetate is in the chelating agent family of medication.[2] It is a salt of edetate with two sodium and one calcium atoms.[3] It works by binding to a number of heavy metals, which renders them almost inert and allows them to leave the body in the urine.[2]
Edetate disodium is a different formulation which does not have the same effects.[2]
Medical use
Sodium calcium edetate's primarily use is to treat lead poisoning,[1] for which it is an alternative to succimer.[2] It is given by slow injection into a vein or into a muscle.[1]
For lead encephalopathy sodium calcium edetate is typically used together with dimercaprol.[2] It may also be used to treat plutonium poisoning.[4] It does not appear to be useful for poisoning by tetra-ethyl lead.[2]
History
Sodium calcium edetate came into medical use in the United States in 1953.[2] It is on the World Health Organization's List of Essential Medicines.[5] As of 2015 in the United States, a course of treatment costs US$50 to US$100.[6]
References
- Stuart MC, Kouimtzi M, Hill SR, eds. (2009). WHO Model Formulary 2008. World Health Organization. pp. 59, 62, 65. hdl:10665/44053. ISBN 9789241547659.
- "Edetate Calcium Disodium". The American Society of Health-System Pharmacists. Archived from the original on 16 January 2017. Retrieved 8 January 2017.
- Kasture, A.V., Dr. (2008). Pharmaceutical Chemistry. I. Pragati Books Pvt. Ltd. p. 16.11. ISBN 9788185790121. Archived from the original on 16 January 2017.
- Flanagan, Robert; Jones, Alison; Maynard, Robert L. (2003). Antidotes: Principles and Clinical Applications. CRC Press. p. 47. ISBN 9780203485071. Archived from the original on 16 January 2017.
- World Health Organization model list of essential medicines: 21st list 2019. Geneva,CH: World Health Organization. 2019. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- Hamilton, Richart (2015). Tarascon Pocket Pharmacopoeia 2015 (annual) (Deluxe Lab-Coat ed.). Jones & Bartlett Learning. p. 471. ISBN 9781284057560.
External links
- "Sodium calcium edetate". U.S. National Library of Medicine. Drug Information Portal. U.S. National Institutes of Health.