Salinomycin
Salinomycin is an antibacterial and coccidiostat ionophore therapeutic drug.
 | |
| Names | |
|---|---|
| IUPAC name
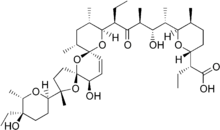
(2R)-2-[(5S,6R)-6-[(1S,2S,3S,5R)-5-[(2S,5R,7S,9S,10S,12R,15R)-2-[(2R,5R,6S)-5-ethyl-5-hydroxy-6-methyl-2-tetrahydropyranyl]-15-hydroxy-2,10,12-trimethyl-1,6,8-trioxadispiro[4.1.5^{7}.3^{5}]pentadec-13-en-9-yl]-2-hydroxy-1,3-dimethyl-4-oxoheptyl]-5-methyl-2-tetrahydropyranyl]butanoic acid | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.052.974 |
| E number | E716 (antibiotics) |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C42H70O11 | |
| Molar mass | 751.00 g/mol |
| Pharmacology | |
| QP51AH01 (WHO) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Antibacterial activity
Salinomycin and its derivatives exhibit high antimicrobial activity against Gram-positive bacteria, including the most problematic bacteria strains such as methicillin-resistant Staphylococcus aureus and methicillin-resistant Staphylococcus epidermidis, and Mycobacterium tuberculosis. Salinomycin is inactive against fungi such as Candida and Gram-negative bacteria. [1]
Cancer research
Pre-clinical
Salinomycin has been shown by Piyush Gupta et al. of the Massachusetts Institute of Technology and the Broad Institute to kill breast cancer stem cells in mice at least 100 times more effectively than the anti-cancer drug paclitaxel. The study screened 16,000 different chemical compounds and found that only a small subset, including salinomycin and etoposide, targeted cancer stem cells responsible for metastasis and relapse.[2][3][4][5]
The mechanism of action by which salinomycin kills cancer stem cells remains unknown, but is thought to be due to its action as a potassium ionophore due to the detection of nigericin in the same compound screen. Studies performed in 2011 showed that salinomycin could induce apoptosis of human cancer cells. Promising results from a few clinical pilot studies reveal that salinomycin is able to effectively eliminate cancer stem cells and to induce partial clinical regression of heavily pretreated and therapy-resistant cancers. The ability of salinomycin to kill both cancer stem cells and therapy-resistant cancer cells may define the compound as a novel and an effective anticancer drug.[6][7] It has been also shown that salinomycin and its derivatives exhibit potent antiproliferative activity against the drug-resistant cancer cell lines.[8][9] Salinomycin is the key compound in the pharmaceutical company Verastem's efforts to produce an anti-cancer-stem-cell drug.
Use in agriculture
Salinomycin is used in chicken feed as a coccidiostat.
Biosynthesis
A team from the University of Cambridge has cloned and sequenced the biosynthetic cluster responsible for salinomycin production, from Streptomyces albus DSM 41398.[10] This has shown that the polyketide backbone of salinomycin is synthesised on an assembly line of nine polyketide synthase) multienzymes. Furthermore, the cluster contains genes involved in oxidative cyclization including salC (epoxidase) and salBI/BII/BIII (epoxide hydrolase) genes. The cluster also contains genes suspected to be involved in self-resistance, export, precursor supply and regulation. The cluster contains a NRPS-like carrier protein, SalX, that is suspected to tether “pre-salinomycin” during oxidative cyclization. By inactivating salC the researchers have demonstrated that salinomycin biosynthesis proceeds via a diene intermediate.
See also
- Narasin a derivative of salinomycin which has an additional methyl group.
- Targeted therapy
References
- M. Antoszczak; et al. (2014). "Synthesis, Anticancer and Antibacterial Activity of Salinomycin N-Benzyl Amides". Molecules. 19 (12): 19435–19459. doi:10.3390/molecules191219435. PMC 6271077. PMID 25429565.
- "Drug shows cancer stem cells not invulnerable". New Scientist. 2009-08-13.
- "New method takes aim at aggressive cancer cells". Broad Communications. Broad Institute. 2009-08-13. Retrieved 2009-08-13.
- Gupta, P.; Onder, Tamer T.; Jiang, Guozhi; Tao, Kai; Kuperwasser, Charlotte; Weinberg, Robert A.; Lander, Eric S.; et al. (2009-08-13). "Identification of selective inhibitors of cancer stem cells by high-throughput screening". Cell. 138 (4): 645–59. doi:10.1016/j.cell.2009.06.034. PMC 4892125. PMID 19682730.
- Adam Huczynski (2012). "Salinomycin – a New Cancer Drug Candidate". Chemical Biology & Drug Design. 79 (3): 235–238. doi:10.1111/j.1747-0285.2011.01287.x. PMID 22145602.
- C. Naujokat, R. Steinhart "Salinomycin as a Drug for Targeting Human Cancer Stem Cells”, Journal of Biomedicine and Biotechnology, Volume 2012 (2012), Article ID 950658, doi: 10.1155/2012/950658, open access review article
- A. Huczyński, ”Polyether ionophores—promising bioactive molecules for cancer therapy”, Bioorganic & Medicinal Chemistry Letters, 2012,22, 7002-7010,doi:10.1016/j.bmcl.2012.09.046, open access review article
- A. Huczyński, J. Janczak, M. Antoszczak, J. Wietrzyk, E. Maj, B. Brzezinski, ” Antiproliferative activity of salinomycin and its derivatives”, Bioorganic & Medicinal Chemistry Letters, 2012, 22, 7146-7150,doi:10.1016/j.bmcl.2012.09.068,
- Antoszczak, Michal; Huczynski, Adam (2015). "Anticancer Activity of Polyether Ionophore-Salinomycin". Anti-Cancer Agents in Medicinal Chemistry. 15 (5): 575–591. doi:10.2174/1871520615666150101130209. PMID 25553435.review article
- Yurkovich, Marie E.; Tyrakis, Petros A.; Hong, Hui; Sun, Yuhui; Samborskyy, Markiyan; Kamiya, Kohei; Leadlay, Peter F.; et al. (2011-11-11). "A Late-Stage Intermediate in Salinomycin Biosynthesis Is Revealed by Specific Mutation in the Biosynthetic Gene Cluster". ChemBioChem. 13 (1): 66–71. doi:10.1002/cbic.201100590. PMID 22076845.