SYBR Safe
SYBR Safe is a cyanine dye[1] used as a nucleic acid stain in molecular biology.[2] SYBR Safe is one of a number of SYBR dyes made by the Life Technologies Corporation. SYBR Safe binds to DNA. The resulting DNA-dye-complex absorbs blue light (λmax = 509 nm) and emits green light (λmax = 524 nm).
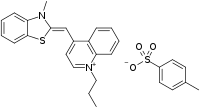 | |
| Names | |
|---|---|
| IUPAC name
(Z)-4-((3-Methylbenzo[d]thiazol-2(3H)-ylidene)methyl)-1-propylquinolin-1-ium 4-methylbenzenesulfonate | |
| Identifiers | |
| Properties | |
| C28H28N2O3S2 | |
| Molar mass | 504.66 g·mol−1 |
| Solubility | Soluble in dimethylsulfoxide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Safety
SYBR Safe is marketed as a safer alternative to ethidium bromide.[3] However, as the molecule itself is quite a bit larger than ethidium bromide, it does not bind to the column of a gel extraction as easily, making it less efficient when trying to clone a DNA fragment into a plasmid. SYBR Safe has a very similar structure to thiazole orange,[4][5] which has a methyl group attached to the charged nitrogen, whereas SYBR Safe has an N-propyl group. Thiazole Orange has been shown to be three to four times less mutagenic than ethidium bromide whereas SYBR Safe is four to five times less mutagenic.[6] Additionally, according to the Life Technologies website, SYBR Safe is not lethal in rats at doses as high as 5 g/kg, and rats don't show symptoms of acute toxicity. Thiazole orange does show toxicity at this dose.
Similar cyanine dyes
- TO (Thiazole Orange)
- SYBR Green I
- SYBR Green II
- SYBR Gold
- YO (Oxazole Yellow)
- PG (PicoGreen)
See also
- GelGreen - competing product with a different molecular structure and size
Notes and references
- Evenson WE; Boden LM; Muzikar KA; O'Leary DJ (2012). "1H and 13C NMR Assignments for the Cyanine Dyes SYBR Safe and Thiazole Orange". Journal of Organic Chemistry. 77 (23): 10967–10971. doi:10.1021/jo3021659. PMID 23137048.
- it is most commonly used as a DNA stain in agarose gel electrophoresis
- Haines, Alicia M.; Tobe, Shanan S.; Kobus, Hilton J.; Linacre, Adrian (March 2015). "Properties of nucleic acid staining dyes used in gel electrophoresis". Electrophoresis. 36 (6): 941–944. doi:10.1002/elps.201400496.
- O'Neil, Casey S.; Beach, Jacie L.; Gruber, Todd D. (June 2018). "Thiazole orange as an everyday replacement for ethidium bromide and costly DNA dyes for electrophoresis". Electrophoresis. 39 (12): 1474–1477. doi:10.1002/elps.201700489.
- Evenson, William E.; Boden, Lauren M.; Muzikar, Katy A.; O’Leary, Daniel J. (27 November 2012). "1H and 13C NMR Assignments for the Cyanine Dyes SYBR Safe and Thiazole Orange". The Journal of Organic Chemistry. 77 (23): 10967–10971. doi:10.1021/jo3021659. PMID 23137048.
- Evenson WE; Boden LM; Muzikar KA; O'Leary DJ (2012). "1H and 13C NMR Assignments for the Cyanine Dyes SYBR Safe and Thiazole Orange". Journal of Organic Chemistry. 77 (23): 10967–10971. doi:10.1021/jo3021659. PMID 23137048.