SU-8 photoresist
SU-8 is a commonly used epoxy-based negative photoresist. Negative refers to a photoresist whereby the parts exposed to UV become cross-linked, while the remainder of the film remains soluble and can be washed away during development.
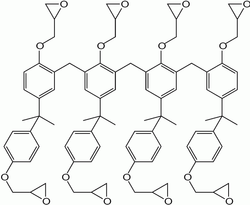
As shown in the structural diagram, SU-8 derives its name from the presence of 8 epoxy groups. This is a statistical average per moiety. It is these epoxies that cross-link to give the final structure.
It can be made into a viscous polymer that can be spun or spread over a thickness ranging from below 1 micrometer up to above 300 micrometers, or Thick Film Dry Sheets (TFDS) for lamination up to above 1 millimetre thick. Up to 500 µm the resist can be processed with standard contact lithography.[1] Above 500 µm absorption leads to increasing sidewall undercuts and poor curing at the substrate interface. It can be used to pattern high aspect ratio structures. An aspect ratio of (> 20) has been achieved with the solution formulation[2] and (> 40) has been demonstrated from the dry resist.[3] Its maximum absorption is for ultraviolet light with a wavelength of the i-line: 365 nm (it is not practical to expose SU-8 with g-line ultraviolet light). When exposed, SU-8's long molecular chains cross-link causing the polymerisation of the material. SU-8 series photoresists use gamma-butyrolactone or cyclopentanone as the primary solvent.
SU-8 was originally developed as a photoresist for the microelectronics industry, to provide a high-resolution mask for fabrication of semiconductor devices.
It is now mainly used in the fabrication of microfluidics (mainly via soft lithography, but also with other imprinting techniques such as nanoimprint lithography[4]) and microelectromechanical systems parts. It is proven to be a biocompatible material [5] and is often used in bio-MEMS for life science applications.[6]
Composition and processing
SU-8 is composed of Bisphenol A Novolac epoxy that is dissolved in an organic solvent (gamma-butyrolactone GBL or cyclopentanone, depending on the formulation) and up to 10 wt% of mixed Triarylsulfonium/hexafluoroantimonate salt as the photoacid generator).[7]
SU-8 absorbs light in the UV region, allowing fabrication of relatively thick (hundreds of micrometers) structures with nearly vertical side walls. The fact that a single photon can trigger multiple polymerizations makes the SU-8 a chemically amplified resist which is polymerized by photoacid generation.[8] The light irradiated on the resist interacts with the salt in the solution creating hexafluoroantimonic acid that then protonates the epoxides groups in the resin monomers. The monomer are thus activated but the polymerization will not proceed significantly until the temperature is raised as part of the post expose bake. It is at this stage that the epoxy groups in the resin cross-link to form the cured structure. When fully cured the high crosslinking degree gives to the resist its excellent mechanical properties.[9]
The processing of SU-8 is similar to other negative resists with particular attention on the control of the temperature in the baking steps. The baking times depend on the SU-8 layer thickness; the thicker the layer, the longer the baking time. The temperature is controlled during the baking in order to reduce stress formation in the thick layer (leading to cracks) as the solvent evaporates.
The soft bake is the most important of the bake steps for stress formation. It is performed after spinning. Its function is to remove the solvent from the resist and make the layer solid. Typically at least 5% of the solvent remains in the layer after the soft bake, however the thicker the coating, the harder it becomes to remove the solvent, as evaporating solvent through thick layers becomes increasingly difficult with coating thickness. The bake is performed on a programmable hot plate to reduce the skinning effect of solvent depletion at the surface creating a dense layer which makes the remainder of the solvent more difficult to remove. In order to reduce stress, the bake procedure is generally a two step process made up of holding at 65 °C before ramping to 95 °C and holding again for a time dependent on the layer thickness. The temperature is then lowered slowly to room temperature.
When dry films are used, the photoresist is laminated rather than spin-coated. As this formulation is essentially solventless (less than 1% solvent remaining), it does not require a soft bake step and does not suffer stress or skinning. For enhanced adhesion, a post lamination bake can be added. This step is carried out in a similar way to the solution based resist - i.e. holding at 65 °C then 95 °C, the time dependent on film thickness.
After this stage the SU-8 layer can now be exposed, Typically this is through a photomask with an inverse pattern, as the resist is negative. The exposure time is a function of exposure dose and film thickness. After exposure the SU-8 needs to be baked again to complete the polymerization. This baking step is not as critical as the prebake but the rising of the temperature (again to 95 °C) needs to be slow and controlled. At this point the resist is ready to be developed.
The main developer for SU-8 is 1-methoxy-2-propanol acetate.[10] Development time is primarily a function of SU-8 thickness.
After exposing and developing, its highly cross-linked structure gives it high stability to chemicals and radiation damage - hence the name "resist". Cured cross-linked SU-8 shows very low levels of outgassing in a vacuum.[11] [12] However it is very difficult to remove, and tends to outgas in an unexposed state.[13]
Newer formulations
SU-8 2000 series resists use cyclopentanone for the primary solvent and can be used to create films between 0.5 and 100 µm in thickness. This formulation may offer improved adhesion on some substrates versus the original formulation.[14]
SU-8 3000 series resists also use cyclopentanone for the primary solvent and are designed to be spun into thicker films ranging from 2 to 75 µm in a single coat.[14]
SU-8 GLM2060 series of low-stress photoresist consist of epoxy GBL and silica formulation CTE 14.[15]
SU-8 GCM3060 Series of GERSTELTEC conductive SU8 with nanoparticles of silver.[15]
SU-8 GMC10xx Series of GERSTELTEC colored SU8 Red, Bleau, Green, black and others.[15]
SU-8 GMJB10XX Series of GERSTELTEC low viscosities epoxy for inkjet applications.[15]
SU8 GM10XX Series of Classic GERSTELTEC epoxy.[16]
Its polymerization process proceeds upon photoactivation of a photoacid generator (triarylsulfonium salts, for example) and subsequent post exposure baking. The polymerization process it a cationic chain growth, which takes place by ring opening polymerization of the epoxide groups.
SUEX is a Thick Dry Film Sheet (TDFS) which is a solventless formulation applied by lamination. As this formulation is a dry sheet, there is high uniformity, no edge-bead formation and very little waste. These sheets come in a range of thicknesses from 100 µm to over 1mm.[17] DJMicrolaminates also sell a thinner range, ADEX TFDS, which are available in thicknesses from 5 µm through to 75 µm.[17]
External links
- SU-8: Thick Photo-Resist for MEMS A webpage with a lot of material data and process tricks.
- http://www.gersteltec.ch/
- Microchem data sheet
- SU 8 Information Provides information on how to use SU 8 to create desired thicknesses.
- SU-8 Spin Speed Calculator Selects a SU-8 type and calculates RPM for a given thickness.
- Suppliers: The solution based SU-8 can be obtained from Microchem or Gersteltec ; the SUEX dry sheets are obtained from DJ Microlaminates , formerly known as DJ Devcorp
References
- "SU-8 Resists: FAQs". MicroChem. Archived from the original on 17 May 2009. Retrieved 21 Jul 2011.
- Liu J, Cai B, Zhu J, et al. (2004). "Process research of high aspect ratio microstructure using SU-8 resist". Microsyst. Technol. 10 (4): 265–8. doi:10.1007/s00542-002-0242-2.
- Johnsona DW, Goettertb J, Singhb V, et al. (2012). "SUEX Dry Film Resist – A new Material for High Aspect Ratio Lithography" (PDF). Louisiana State University Proceedings.
- Greener J, Li W, Ren J, et al. (February 2010). "Rapid, cost-efficient fabrication of microfluidic reactors in thermoplastic polymers by combining photolithography and hot embossing". Lab on a Chip. 10 (4): 522–4. doi:10.1039/B918834G. PMID 20126695.
- Matarèse BF, Feyen PL, Falco A, Benfenati F, Lugli P, deMello JC (April 2018). "Use of SU8 as a stable and biocompatible adhesion layer for gold bioelectrodes". Scientific Reports. 8 (1): 5560. doi:10.1038/s41598-018-21755-6. PMC 5882823. PMID 29615634.
- Arscott S (October 2014). "SU-8 as a material for lab-on-a-chip-based mass spectrometry". Lab on a Chip. 14 (19): 3668–89. doi:10.1039/C4LC00617H. PMID 25029537.
- "NANOTM SU-8: Negative Tone Photoresist - formulations 50-100" (PDF). Microchem.com. 2011. Retrieved 12 Jun 2019.
- del Campo A, Greiner C (2007). "SU-8: a photoresist for high-aspect-ratio and 3D submicron lithography". J. Micromech. Microeng. 17 (6): R81–R95. doi:10.1088/0960-1317/17/6/R01.
- Martinez-Duarte R, Madou M (2011). "SU-8 Pholithography and its impact on microfluidics". In Mitra SK, Chakraborty S (eds.). Microfluidics and Nanofluidics Handbook: Fabrication, Implementation and Applications (1st ed.). New York: CRC Press. pp. 231–268. ISBN 9781138072381.
- "SU-8 Developer". Lambers Wiki (Material Safety Data Sheet). 2005. Archived from the original on 11 December 2017. Retrieved 12 Jun 2019.
- "SU-8 photosensitive epoxy". 2003. Archived from the original on 30 May 2012. Retrieved 12 Jun 2019.
- Melai J, Salm C, Wolters R, et al. (2009). "Qualitative and quantitative characterization of outgassing from SU-8". Microelectronic Engineering. 86 (4–6): 761–764. doi:10.1016/j.mee.2008.11.008.
- "SU-8 Photoresist Processing" (PDF). engineering.tufts.edu. 2007. Archived from the original (PDF) on 9 November 2009. Retrieved 12 Jun 2019.
- "SU-8 2000 Permanent Epoxy Negative Photoresist Processing Guidelines" (PDF). Microchem. Archived from the original (PDF) on 15 April 2017.
- "SU-8 Functional Polymer". Gersteltec Engineering Solutions. Retrieved 12 Jun 2019.
- "SU8". Gersteltec Engineering Solutions. Retrieved 12 Jun 2019.
- "SUEX". djmicrolaminates.com. Retrieved 15 Feb 2017.