Ruddlesden-Popper phase
Ruddlesden-Popper (RP) phases are type of perovskite structure that consists of two-dimensional perovskite-like slabs interleaved with cations. The general formula of RP phase is An+1BnX3n+1, where A and B are cations, X is an anion (e.g., oxygen), and n is the number of the layers of octahedra in the perovskite-like stack.[1] Generally, it has a phase structure that results from the intergrowth of perovskite-type and NaCl-type (i.e., rocksalt-type) structures.
The structure was named after S.N. Ruddlesden and P. Popper, who first synthesized and described the structure in 1957.[2][3]
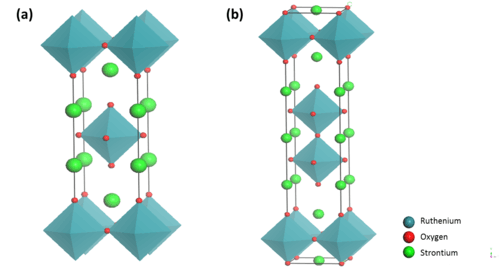
Crystal Structure
The general RP formula An+1BnX3n+1 can be represented as An-1A’2BnX3n+1, where A and A’ represent alkali, alkaline earth, or rare earth metal while B refers to transition metal. The A cations are located in the perovskite layer and have a 12-fold cuboctahedral coordination to the anions (CN = 12). The A’ cations have a coordination number of 9 (CN = 9) and are located at the boundary between the perovskite layer and an intermediate block layer. The B cations are located inside the anionic octahedral, pyramids and squares.[4]
Synthesis
The first series of Ruddlesden-Popper phase, Sr2TiO4, Ca2MnO4 and SrLaAlO4 were confirmed by powder X-ray diffraction (PXRD) in 1957.[2] These compounds were formed by heating up the appropriate oxide or carbonate, in the molecular proportion.
In recent years, interest on perovskite-like structure has been growing and synthetic methods for this compound have been further developed. Alternative to the conventional solid state method, chimie douce or soft chemistry solid-state technique has been utilized to synthesize this class of material. These soft chemistry solid-state techniques include ion-exchange reactions of layered perovskites, ion exchange reactions involving interlayer structural units, topochemical condensation reactions and other techniques such as intercalation-deintercalation reactions and multistep intercalation reactions of layer perovskite.[5]
Applications
Similar to its parent perovskite structure, Ruddlesden-Popper phases possess interesting properties such as colossal magnetoresistance, superconductivity, ferroelectricity, and catalytic activity.
The Ruddlesden-Popper phase LaSr3FeO10 is an example of a layered perovskite that finds its application in the rechargeable metal-air battery.[6] Due to the layered structure of the Ruddlesden-Popper phase, the oxygen located in between the layered perovskite is easily removable. The easily removable oxygen is essential to increase the efficiency of Oxygen Evolution Reaction (OER) and Oxygen Reduction Reaction (ORR). In the metal-air battery, OER is a process of charging reaction at the air electrode, while ORR is a process of discharging reaction.
References
- Wells, A.F. (1984). Structural Inorganic Chemistry. Oxford: Clarendon. p. 602. ISBN 0-19-855370-6.
- Ruddlesden, S.N.; Popper, P. (1958). "The compound Sr3Ti2O7 and its structure". Acta Crystallogr. 11: 54–55. doi:10.1107/S0365110X58000128.
- Ruddlesden, S.N.; Popper, P. (1957). "New compounds of the K2NiF4 type". Acta Crystallogr. 10: 538–539. doi:10.1107/S0365110X57001929.
- Beznosikov, B.V.; Aleksandrov, K.S. (2000). "Perovskite-like crystals of the Ruddlesden-Popper series". Crystallography Reports. 45: 792–798. doi:10.1134/1.1312923.
- Schaak, R.E.; Mallouk, T.E. (2002). "Perovskites by Design: A Toolbox of Solid-State Reactions". Chemistry of Materials. 14: 1455–1471. doi:10.1021/cm010689m.
- Takeguchi, T.; Yamanaka, T.; Takahashi, H.; Watanabe, H.; Kuroki, T.; Nakanishi, H.; Orikasa, Y.; Uchimoto, Y.; Takano, H.; Ohguri, N.; Matsuda, M.; Murota, T.; Uosaki, K.; Ueda, W. (2013). "Layered Perovskite Oxide: A Reversible Air Electrode for Oxygen Evolution/Reduction in Rechargeable Metal-Air Batteries". Journal of the American Chemical Society. 135: 11125–11130. doi:10.1021/ja403476v. PMID 23802735.