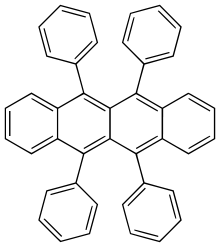
Rubrene
Rubrene (5,6,11,12-tetraphenyltetracene) is a red colored polycyclic aromatic hydrocarbon. Rubrene is used as a sensitiser in chemoluminescence and as a yellow light source in lightsticks.
 | |
 | |
 | |
| Names | |
|---|---|
| IUPAC name
5,6,11,12-Tetraphenyltetracene | |
| Other names
5,6,11,12-Tetraphenylnaphthacene, rubrene | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.007.494 |
| EC Number |
|
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C42H28 | |
| Molar mass | 532.7 g/mol |
| Melting point | 315 °C (599 °F; 588 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Electronic properties
As an organic semiconductor, the major application of rubrene is in organic light-emitting diodes (OLEDs) and organic field-effect transistors, which are the core elements of flexible displays. Single-crystal transistors can be prepared using crystalline rubrene, which is grown in a modified zone furnace on a temperature gradient. This technique, known as physical vapor transport, was introduced in 1998.[1][2]
Rubrene holds the distinction of being the organic semiconductor with the highest carrier mobility, reaching 40 cm2/(V·s) for holes. This value was measured in OFETs prepared by peeling a thin layer of single-crystalline rubrene and transferring to a Si/SiO2 substrate.[3]
Crystal structure
Several polymorphs of rubrene are known. Crystals grown from vapor in vacuum can be monoclinic,[4] triclinic,[5] and orthorhombic motifs.[6] Orthorhombic crystals (space group Bbam) are obtained in a closed system in a two-zone furnace at ambient pressure.[7]
Synthesis
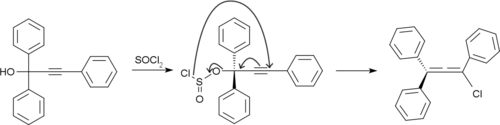
Rubrene is prepared by treating 1,1,3-triphenylprop-2-yne-1-ol with thionyl chloride.[8]
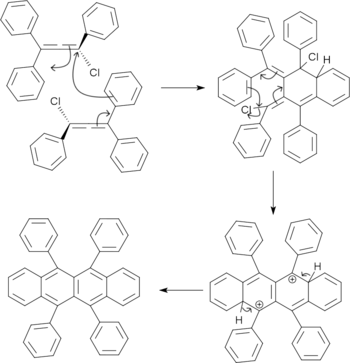
The resulting chloroallene undergoes dimerization and dehydrochlorination to give rubrene.[9]
Redox properties
Rubrene, like other polycyclic aromatic molecules, undergoes redox reactions in solution. It oxidizes and reduces reversibly at 0.95 V and −1.37 V, respectively vs SCE. When the cation and anion are co-generated in an electrochemical cell, they can combine with annihilation of their charges, but producing an excited rubrene molecule that emits at 540 nm. This phenomenon is called electrochemiluminescence.[10]
References
| Wikimedia Commons has media related to Rubrene. |
- Laudise, R.A; Kloc, Ch; Simpkins, P.G; Siegrist, T (1998). "Physical vapor growth of organic semiconductors". Journal of Crystal Growth. 187 (3–4): 449. Bibcode:1998JCrGr.187..449L. doi:10.1016/S0022-0248(98)00034-7.
- Jurchescu, Oana Diana (2006) "Low Temperature Crystal Structure of Rubrene Single Crystals Grown by Vapor Transport" in Molecular organic semiconductors for electronic devices, PhD thesis Rijksuniversiteit Groningen.
- Hasegawa, Tatsuo and Takeya, Jun (2009). "Organic field-effect transistors using single crystals". Sci. Technol. Adv. Mater. 10 (2): 024314. Bibcode:2009STAdM..10b4314H. doi:10.1088/1468-6996/10/2/024314. PMC 5090444. PMID 27877287.CS1 maint: multiple names: authors list (link)
- Taylor, W. H. (1936). "X-ray measurements on diflavylene, rubrene, and related compounds". Zeitschrift für Kristallographie. 93: 151. doi:10.1524/zkri.1936.93.1.151.
- Akopyan, S. A.; Avoyan, R. L. and Struchkov, Yu. T. Z. Strukt. Khim. 3, 602 (1962)
- Henn, D. E. & Williams, W. G. (1971). "Crystallographic data for an orthorhombic form of rubrene". J. Appl. Cryst. 4 (3): 256. doi:10.1107/S0021889871006812.
- Bulgarovskaya, I.; Vozzhennikov, V.; Aleksandrov, S.; Belsky, V. (1983). Latv. PSR Zinat. Akad. Vestis, Fiz. Teh. Zinat. Ser. 4. 53: 115
- Furniss, B. Vogel's Textbook of Practical Organic Chemistry (5th ed.). pp. 840–841.
- Furniss, B. Vogel's Textbook of Practical Organic Chemistry (5th ed.). pp. 844–845.
- Richter, M. M. (2004). "Electrochemiluminescence (ECL)". Chemical Reviews. 104 (6): 3003–36. doi:10.1021/cr020373d. PMID 15186186.