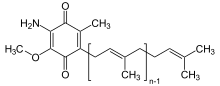
Rhodoquinone
 | |
| Names | |
|---|---|
| IUPAC name
2-[(2E,6E,10E,14E,18E,22E,26E,30E,34E)-3,7,11,15,19,23,27,31,35,39-Decamethyltetraconta-2,6,10,14,18,22,26,30,34,38-decaenyl]-5,6-dimethoxy-3-methylcyclohexa-2,5-diene-1,4-dione | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C58H89NO3 | |
| Molar mass | 848.354 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Background
Rhodoquinone (RQ) is a modified ubiquinone-like molecule that is an important cofactor used in anaerobic energy metabolism by many organisms. Recently, it has gained attention as a potential anthelmintic drug target due to the fact that parasitic hosts do not synthesize or use this cofactor. Because this cofactor is used in low oxygen environments, many helminth-like organisms have adapted to survive host environments such as the areas within the gastrointestinal tracks.[1][2]
Theorized Biosynthesis
Currently the biosynthesis of RQ is still being debated, but there are two main biosynthetic pathways that are being researched. First, is the need for the organism to produce Ubiquinone (UQ) so that the amine group can be added onto the quinone ring. Second, is that RQ can be synthesized without any UQ within the organism by using tryptophan metabolites instead.
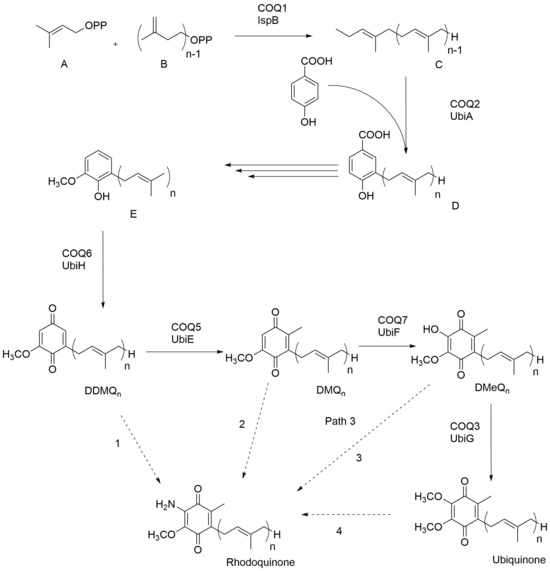
In the case of the prokaryotic organism P. rubrum, RQ seems to be synthesized by addition of an amine group to a pre-existing UQ; thus UQ needs to be present as a precursor before RQ can be made. Figure 1 uses ‘n’ to represent the number of isoprene units between various organisms. Dimethylallyl diphosphate A and isopentyl diphosphate B come together to form polyisoprenyl diphosphate C. With the addition of p-hydroxybenzoic acid, the product that arises is 3-polyprenyl-4-hydroxybenzoic acid D. The next three steps of synthesis varies between different organisms, but molecule E is made across all organisms and through oxidation, demethyldemethoxyubiquinone (DDMQ) is eventually formed. Rhodoquinone has been theorized to be synthesized from DDMQn, DMQn, DMeQn, and ubiquinone, as shown with the dashed arrows. Recent studies have shown that path D- RQ biosynthesis via UQ, seems to be the most likely route.[3]
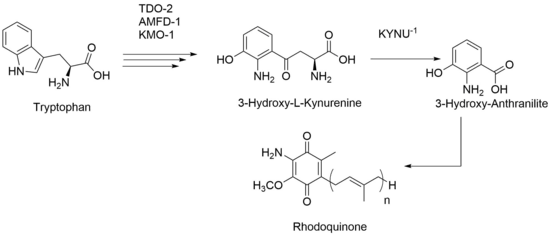
Research in C. elegans, has shown an alternative path for production of RQ. Even after knocking out all UQ production, RQ is still present within those mutant strains. Based on this data, RQ production is not solely based on UQ-like molecule and instead can be made via tryptophan metabolites. Therefore, the amine group that has been previously proposed to be added in late stages of synthesis is instead always present throughout intermediate stages. With this proposed biosynthesis, the kynurenine pathway still needs to be upregulated, and activity from certain genes like KYNU-1, an enzyme that catalyzes production of 3-Hydroxy-L-Kynurenine to 3-Hydroxy-Anthranilite, needs to be upheld.[4]
References
- "[Prevention and control of schistosomiasis and soil-transmitted helminthiasis:report of a WHO expert committee]". World Health Organization. 49 (3): 57. June 2012. ISBN 9241209127.
- Stairs, C (April 2018). "[Microbial eukaryotes have adapted to hypoxia by horizontal acquisitions of a gene involved in rhodoquinone biosynthesis]". eLife. doi:10.7554/eLife.34292.
- Brajcich, B (December 2009). "[Evidence that Ubiquinone Is a Required Intermediate for Rhodoquinone Biosynthesis in Rhodospirillum rubrum]". Journal of Bacteriology. 192 (2): 436–445. doi:10.1128/JB.01040-09.
- Del Borrello, S (June 2019). "[Rhodoquinone biosynthesis in C. elegans requires precursors generated by the kynurenine pathway]". eLife. doi:10.7554/eLife.48165.