Rhodolith
Rhodoliths (from Greek for red rocks) are colorful, unattached, branching, crustose, benthic marine red algae that resemble coral. Rhodolith beds create biogenic habitat for diverse benthic communities. The rhodolithic growth habit has been attained by a number of unrelated coralline red algae,[1] organisms that deposit calcium carbonate within their cell walls to form hard structures or nodules that closely resemble beds of coral.
.jpg)
Unlike coral, rhodoliths do not attach themselves to the rocky seabed. Rather, they roll like tumbleweeds along the seafloor until they become too large in size to be mobilised by the prevailing wave and current regime. They may then become incorporated into a semi-continuous algal mat. While corals are animals that are both autotrophic (photosynthesize via their symbionts) or heterotrophic (filter plankton from the water for food), rhodoliths produce energy solely through photosynthesis (i.e. they can only grow and survive in the shallow photic zone of the ocean).
Common rhodolith species include Lithophyllum margaritae, Lithothamnion muellerii, and Neogoniolithon trichotomum.[2] Scientists believe rhodoliths have been present in the world's oceans since at least the Eocene epoch, some 55 million years ago.[3]
Overview
Rhodoliths (including maërl) have been defined as calcareous nodules composed of more than 50% of coralline red algal material and consisting of one to several coralline species growing together.[4][5]
Habitat

Rhodolith beds have been found throughout the world's oceans, including in the Arctic near Greenland, off eastern Australia[6] and in waters off British Columbia, Canada. Globally, rhodoliths fill an important niche in the marine ecosystem, serving as a transition habitat between rocky areas and barren, sandy areas. Rhodoliths provide a stable and three-dimensional habitat onto which a wide variety of species can attach, including other algae, commercial species such as clams and scallops, and true corals.[3] Living rhodolith beds are widely distributed throughout the Gulf of California, Mexico.[7] Rhodoliths are resilient to a variety of environmental disturbances, but can be severely impacted by harvesting of commercial species.
Rhodoliths come in many shapes, including laminar, branching and columnar growth forms.[8] In shallow water and high-energy environments, rhodoliths are typically mounded, thick or unbranched; branching is also rarer in deeper water, and most profuse in tropical, mid-depth waters.[1]
 Rhodoliths on the northern shore of Fuerteventura
Rhodoliths on the northern shore of Fuerteventura- A fossilised rhodolith from the Messinian of southern Spain
Geological significance
Rhodoliths are a common feature of modern and ancient carbonate shelves worldwide. Rhodolith communities contribute significantly to the global calcium carbonate budget, and fossil rhodoliths are commonly used to obtain paleoecologic and paleoclimatic information.[9] Under the right circumstances, rhodoliths can be the main carbonate sediment producers, often forming rudstone or floatstone beds consisting of large pieces of rhodoliths in grainy matrix.
Climate change and the rhodolith holobiont
Rhodoliths are significant photosynthesizers, calcifiers, and ecosystem engineers, which raises an issue about how they might respond to ocean acidification.[11]
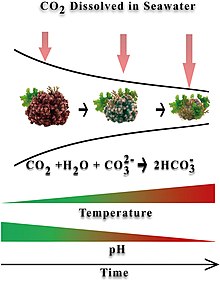
Changes in ocean carbonate chemistry driven by increasing anthropogenic carbon dioxide emissions promotes ocean acidification. Increasing the ocean carbon dioxide uptake results in increases in pCO2 (the partial pressure of carbon dioxide in the ocean) as well as lower pH levels and a lower carbonate saturation in the seawater. These affect the calcification process.[12] Organisms like rhodoliths accrete carbonate as part of their physical structure, since precipitating CaCO3 would be less efficient.[13][14] Ocean acidification presents a threat by potentially affecting their growth and reproduction.[15][16] Coralline algae are particularly sensitive to ocean acidification because they precipitate high magnesium-calcite carbonate skeletons, the most soluble form of CaCO3.[17][18][11]
Calcification rates in coralline algae are thought to be directly related to their photosynthetic rates, but it is not clear how a high-CO2 environment might affect rhodoliths.[19] Elevated CO2 levels might impair biomineralization due to decreased seawater carbonate (CO2−
3) availability as pH falls, but photosynthesis could be promoted as the availability of bicarbonate (HCO−
3) increases.[20] This would result in a parabolic relationship between declining pH and coralline algal fitness, which could explain why varied responses to declining pH and elevated pCO2 have been recorded to date.[21][11]
The widespread distribution of rhodoliths hints at the resilience of this algal group, which have persisted as chief components of benthic marine communities through considerable environment changes over geologic times.[22][11]
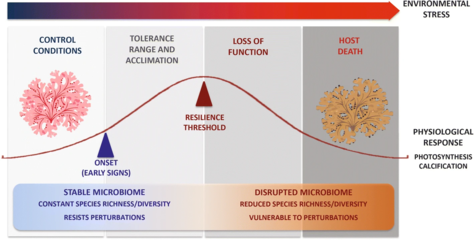
In 2018 the first metagenomic analysis of live rhodoliths was published. Whole genome shotgun sequencing was performed on a variety of rhodolith bed constituents. This revealed a stable live rhodolith microbiome thriving under elevated pCO2 conditions, with positive physiological responses such as increased photosynthetic activity and no calcium carbonate biomass loss over time. However, the seawater column and coralline skeleton biofilms showed significant microbial shifts. These findings reinforce the existence of a close host-microbe functional entity, where the metabolic crosstalk within the rhodolith as a holobiont could be exerting reciprocal influence over the associated microbiome.[11]
While the microbiome associated with live rhodoliths remained stable and resembled a healthy holobiont, the microbial community associated with the water column changed after exposure to elevated pCO2.[11]
See also
References
- Steneck, R. S. (1986). "The Ecology of Coralline Algal Crusts: Convergent Patterns and Adaptative Strategies". Annual Review of Ecology and Systematics. 17: 273–303. doi:10.1146/annurev.es.17.110186.001421. JSTOR 2096997.
- Riosmena-rodríguez, R.; Steller, D.L.; Foster, M.S. (2007). "Prefacio: Trabajos selectos de investigación sobre rodolitos Preface: Selected research papers on rhodoliths" (PDF). Ciencias Marinas. 33 (4). Archived (PDF) from the original on 2012-04-06. Retrieved 2008-05-08.
- Science Daily, September 23, 2004
- Horta, P.A., Riul, P., Amado Filho, G.M., Gurgel, C.F.D., Berchez, F., Nunes, J.M.D.C., Scherner, F., Pereira, S., Lotufo, T., Peres, L. and Sissini, M. (2016) "Rhodoliths in Brazil: Current knowledge and potential impacts of climate change". Brazilian Journal of Oceanography, 64(SPE2): 117–136. doi:10.1590/S1679-875920160870064sp2.

- Bosellini, A. and Ginsburg, R.N. (1971) "Form and internal structure of recent algal nodules (rhodolites) from Bermuda". The Journal of Geology, 79(6): 669–682. doi:10.1086/627697.
- Harris, P.T., Tsuji, Y., Marshall, J.F., Davies, P.J., Honda, N., Matsuda, H., 1996. Sand and rhodolith-gravel entrainment on the mid- to outer-shelf under a western boundary current: Fraser Island continental shelf, eastern Australia. Marine Geology 129, 313-330.
- source:Diana Steller, Moss Landing Marine Laboratories Archived 2008-11-23 at the Wayback Machine
- Bosence, D.W. (1983) "Description and classification of rhodoliths (rhodoids, rhodolites)". In: Coated grains, pages 217-224, Springer, Berlin. doi:10.1007/978-3-642-68869-0_19. ISBN 9783642688690.
- Hetzinger, S.; Halfar, J.; Riegl, B.; Godinez-Orta, L. (April 2006). "Sedimentology and Acoustic Mapping of Modern Rhodolith Facies on a Non-Tropical Carbonate Shelf (Gulf of California, Mexico)". Journal of Sedimentary Research. 76 (4): 670–682. Bibcode:2006JSedR..76..670H. doi:10.2110/jsr.2006.053.
- IPPC (2014) Climate change 2014 impacts, adaptation, and vulnerability, Part B. ISBN 978-1-107-05816-3
- Cavalcanti, G.S., Shukla, P., Morris, M., Ribeiro, B., Foley, M., Doane, M.P., Thompson, C.C., Edwards, M.S., Dinsdale, E.A. and Thompson, F.L. (2018) "Rhodoliths holobionts in a changing ocean: host-microbes interactions mediate coralline algae resilience under ocean acidification". BMC Genomics, 19(1): 1–13. doi:10.1186/s12864-018-5064-4.

- Millero, F.J., Graham, T.B., Huang, F., Bustos-Serrano, H. and Pierrot, D. (2006) "Dissociation constants of carbonic acid in seawater as a function of salinity and temperature". Marine Chemistry, 100(1–2): 80–94. doi:10.1016/j.marchem.2005.12.001.
- Orr, J.C., Fabry, V.J., Aumont, O., Bopp, L., Doney, S.C., Feely, R.A., Gnanadesikan, A., Gruber, N., Ishida, A., Joos, F. and Key, R.M., 2005. Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms. Nature, 437(7059), pp.681-686. doi:10.1038/nature04095.
- Hoegh-Guldberg, O., Mumby, P.J., Hooten, A.J., Steneck, R.S., Greenfield, P., Gomez, E., Harvell, C.D., Sale, P.F., Edwards, A.J., Caldeira, K. and Knowlton, N., 2007. Coral reefs under rapid climate change and ocean acidification. science, 318(5857), pp.1737-1742. doi:10.1126/science.1152509.
- Kroeker, K.J., Kordas, R.L., Crim, R., Hendriks, I.E., Ramajo, L., Singh, G.S., Duarte, C.M. and Gattuso, J.P., 2013. Impacts of ocean acidification on marine organisms: quantifying sensitivities and interaction with warming. Global change biology, 19(6), pp.1884-1896. doi:10.1111/gcb.12179.
- Riebesell, U. and Gattuso, J.P., 2015. Lessons learned from ocean acidification research. Nature Climate Change, 5(1), pp.12-14. doi:10.1038/nclimate2456.
- Bischoff, W.D., Bishop, F.C. and Mackenzie, F.T. (1983) "Biogenically produced magnesian calcite; inhomogeneities in chemical and physical properties; comparison with synthetic phases". American Mineralogist, 68(11–12): 1183–1188.
- Martin, S. and GATTUSO, J.P., 2009. Response of Mediterranean coralline algae to ocean acidification and elevated temperature. Global Change Biology, 15(8), pp.2089-2100. doi:10.1111/j.1365-2486.2009.01874.x.
- McCoy, S.J. and Kamenos, N.A., 2015. Coralline algae (Rhodophyta) in a changing world: integrating ecological, physiological, and geochemical responses to global change. Journal of Phycology, 51(1), pp.6-24. doi:10.1111/jpy.12262.
- Johnson, M.D., Price, N.N. and Smith, J.E., 2014. Contrasting effects of ocean acidification on tropical fleshy and calcareous algae. PeerJ, 2, p.e411. doi:10.7717/peerj.411.
- Ries, J.B., Cohen, A.L. and McCorkle, D.C., 2009. Marine calcifiers exhibit mixed responses to CO2-induced ocean acidification. Geology, 37(12), pp.1131-1134. doi:10.1130/G30210A.1.
- Weiss A, Martindale RC (2017) "Crustose coralline algae increased framework and diversity on ancient coral reefs". PLoS One, 12: e0181637. doi:10.1371/journal.pone.0181637.
Other references
- Riosmena-Rodríguez R, Nelson W and Aguirre J (Eds.) (2016) Rhodolith/Maërl Beds: A Global Perspective Springer. ISBN 9783319293158.