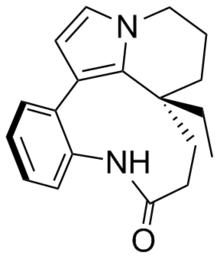
Rhazinilam
Rhazinilam is an alkaloid first isolated in 1965 by Linde from the Melodinus australis plant. It was later isolated from the shrub Rhazya stricta as well as from other organisms.
 | |
| Names | |
|---|---|
| IUPAC name
(3aR)-3a-Ethyl-2,3,3a,4,5,7-hexahydroindolizino[8,1-ef][1]benzazonin-6(1H)-one | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C19H22N2O | |
| Molar mass | 294.398 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Biological activity
Rhazinilam has activity similar to that of colchicine, taxol and vinblastine, acting as a spindle poison.[1]
Total synthesis
Rhazinilam was first synthesized in 1973 by Smith and coworkers, and multiple subsequent times.[2]
Trauner synthesis
Bowie, Alfred L.; Hughes, Chambers C.; Trauner, Dirk. "Concise Synthesis of (±)-Rhazinilam through Direct Coupling". Organic Letters. 7 (23): 5207–5209. doi:10.1021/ol052033v. PMID 16268539.
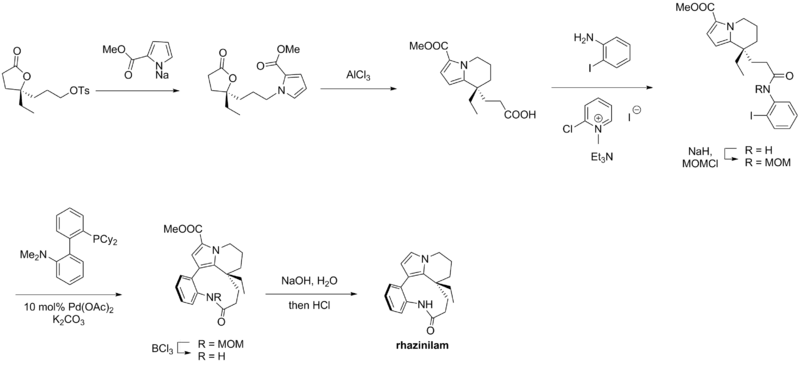 Rhazinilam – Trauner synthesis
Rhazinilam – Trauner synthesis
gollark: I have at least two arch-related memes.
gollark: Nope, I use Arch btw and am contractually obligated to tell you that.
gollark: MacOS *does* probably beat Windows.
gollark: That would be neat, although probably hard to cool and power sensibly since desktop CPUs are very hot.
gollark: At least mine actually has replaceable memory/storage, and adequate cooling.
References
- David, Bruno; Sévenet, Thierry; Thoison, Odile; Awang, Khalijah; Païs, Mary; Wright, Michel; Guénard, Daniel. "Hemisynthesis of rhazinilam analogues: structure - activity relationships on tubulin-microtubule system". Bioorganic & Medicinal Chemistry Letters. 7 (17): 2155–2158. doi:10.1016/S0960-894X(97)00391-0.
- Ratcliffe, A.H.; Smith, G.F.; Smith, G.N. "The synthesis of rhazinilam". Tetrahedron Letters. 14 (52): 5179–5184. doi:10.1016/S0040-4039(01)87657-7.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.