Reticulated foam
Reticulated foam is a very porous, low density solid foam. 'Reticulated' means like a net. Reticulated foams are extremely open foams i.e. there are few, if any, intact bubbles or cell windows. In contrast, the foam formed by soap bubbles is composed solely of intact (fully enclosed) bubbles. In a reticulated foam only the lineal boundaries where the bubbles meet (Plateau borders) remain.
The solid component of a reticulated foam may be an organic polymer like polyurethane, a ceramic or a metal. These materials are used in a wide range of applications where the high porosity and large surface area are needed, including filters, catalyst supports, fuel tank inserts, and loudspeaker covers.
Structure and properties
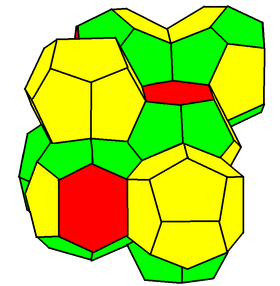
A description of the structure of reticulated foams is still being developed. While Plateau's laws, the rules governing the shape of soap films in foams were developed in the 19th century, a mathematical description of the structure is still debated. The computer-generated Weaire–Phelan structure is the most recent. In a reticulated foam only the edges of the polyhedra remain; the faces are missing. In commercial reticulated foam, up to 98% of the faces are removed. The dodecahedron is sometimes given as the basic unit for these foams,[1] but the most representative shape is a polyhedron with 13 faces.[2][3] Cell size and cell size distribution are critical parameters for most applications. Porosity is typically 95%, but can be as high as 98%.[4] Reticulation affects many of the physical properties of a foam. Typically resistance to compression is decreased while tensile properties like elongation and resistance to tearing are increased.[5]
Production
Robert A. Volz is credited with discovering the first process for making reticulated polyurethane foam in 1956 while working for the Scott Paper Company.[6] Producing reticulated polyurethane foam is a two step procedure: a conventional closed-cell polyurethane foam is produced, then the faces (or "windows") of the cells are removed. The high surface area and lower mass of the cells' faces compared with the cells' struts (or edges) makes them much more susceptible to both combustion and chemical degradation; either filling the closed-cell foam with a combustible gas like hydrogen and igniting it under controlled conditions, or exposing the foam to a sodium hydroxide solution will remove the faces and leave the edges.[7]
Reticulated ceramic foams are made by coating a reticulated polyurethane foam with an aqueous suspension of a ceramic powder then heating the material to first evaporate the water then fuse the ceramic particles and finally to burn off the organic polymer.[4]
Reticulated metal foam can also be made using polyurethane foam as a template similar to its use in ceramic foams. Metals can be vapor deposited onto the polyurethane foam and then the organic polymer burned off.[8]
Applications
Reticulated foams are used where porosity, surface area, low density are important.
- Puppets (such as the bodies/faces/hands of The Muppets)
- Humidifier pads
- Air conditioner filters[9]
- Scrubbers
- Ceramic filters for filtering molten metal[9]
- Vehicle and bacteria filters
- Speaker grills[9]
- Face mask and pads
- Shoe polish and cosmetic applicators[9]
- Ink jet cartridges[9]
- Aquaculture (water purification)[10]
- Anti-slosh filling in fuel tanks for aircraft and race cars[11] For example: A-10 Thunderbolt II[12]
References
- "The "shape" of our foam". Crest Foams. Retrieved 2009-09-30.
- "Structure of random monodisperse foam" (PDF).
- "A new counter-example to Kelvin's conjecture on minimal foams". Cite journal requires
|journal=(help) - Gliganic, Robert (February 21, 2008). "Where Reticulated Polyurethane Foam's a Fit". MachineDesign.com. Retrieved 2009-09-30.
- Blair, E. Allen (1967). Cellular plastics: proceedings of a conference, Natick, Massachusetts, April 13-15, 1966. National Academy of Sciences. p. 141. Retrieved 2010-12-02.
- "Hall of Fame". Polyurethane Foam Association. Retrieved 2009-09-30.
- "Reticulated foam". United Foam. Retrieved 2009-09-30.
- Queheillalt, Douglas T.; Derek D. Hass, David J. Sypeck, Haydn N.G. Wadley, DD.; Sypeck, DJ.; Wadley, HN. (2001). "Synthesis of open-cell metal foams by templated directed vapor deposition" (PDF). Journal of Materials Research. 16 (4): 1028–1036. Bibcode:2001JMatR..16.1028Q. doi:10.1557/JMR.2001.0143.CS1 maint: multiple names: authors list (link)
- Curti, Michael C. "A Multifaceted Foam". Crest Foam Industries. Retrieved 2009-09-30.
- Thomson, Tim (2004). Polyurethanes as specialty chemicals: principles and applications. CRC. p. 99. ISBN 978-0-8493-1857-3. Retrieved 2009-09-30.
- Gliganic, Robert. "The Unique Material for Imaginative Applications". Product Design and Development. Archived from the original on 2013-01-31. Retrieved 2009-09-30.
- Bennett, J. Michael. "Novel Halon Alternative Concepts – Synergistic Development of Public and Private Sectors" (PDF). National Institute of Standards and Technology. p. 3. Retrieved 2009-10-01.