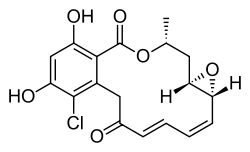
Radicicol
Radicicol, also known as monorden, is a natural product that binds to Hsp90 (Heat Shock Protein 90) and alters its function. HSP90 client proteins play important roles in the regulation of the cell cycle, cell growth, cell survival, apoptosis, angiogenesis and oncogenesis.
 | |
| Names | |
|---|---|
| IUPAC name
(1aR,2E4E,14R,15aR)-8-chloro-9,11-dihydroxy-14-methyl-1a,14,15,15a-tetrahydro-6H-oxireno[e][2]benzoxacyclotetradecine-6,12(7H)-dione | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.170.695 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C18H17ClO6 | |
| Molar mass | 364.78 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Biosynthesis
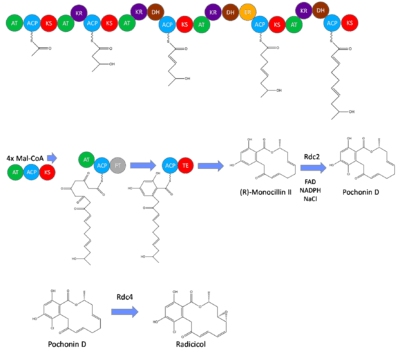
Biosynthesis of Radicicol has been best studied in Pochonia chlamydosporia, in which the majority of the core structure is produced in vivo through iterative type I polyketide synthases.[1] This structure produced is the earliest intermediate in the radicicol biosynthesis, monocillin II. This intermediate is transformed to radicicol through halogenation and epoxide formation performed by RadH and RadP respectively.[2] These enzymes are coded by the genes Rdc2 and Rdc4 in the pathway, and removing either of these results in a product that has the monocillin II core, but does not have either the epoxide or halogen added.[3]
See also
References
- Zhou H, Qiao K, Gao Z, Vederas JC, Tang Y. "Insights into Radicicol Biosynthesis via Heterologous Synthesis of Intermediates and Analogs". Journal of Biological Chemistry. 285: 41412–41421. doi:10.1074/jbc.M110.183574. PMC 3009867. PMID 20961859.
- "Functional Characterization of the Biosynthesis of Radicicol, an Hsp90 Inhibitor Resorcylic Acid Lactone from Chaetomium chiversii". Chemistry. 15: 1328–1338. doi:10.1016/j.chembiol.2008.10.006.
- "Functional Characterization of the Biosynthesis of Radicicol, an Hsp90 Inhibitor Resorcylic Acid Lactone from Chaetomium chiversii". Chemistry. 15: 1328–1338. doi:10.1016/j.chembiol.2008.10.006.
Further reading
- Winssinger N, Barluanga S (January 2007). "Chemistry and biology of resorcylic acid lactones". Chem Commun. 13 (1): 22–36. doi:10.1039/B610344H. Review of the chemistry and biology of resorcylic acid lactones, including radicicol.