RIOK1
Serine/threonine-protein kinase RIO1 is an enzyme that in humans is encode by the RIOK1 gene.[5]
RIOK1 is an atypical protein, which exists in most archaea and eukaryotes. It belongs to the serine/threonine-specific protein kinase family.[6][7]
It has been intensely studied to understand the maturation they promote on small ribosomal subunits (SSU). It is suggested that over-expression or mutations of the RIOK 1 gene may cause mis-regulation of its network (in metazoans - large signaling network at the protein and gene levels via which it stimulates or restricts growth and division in response to nutrient availability). This was observed in primary cancer cells and may contribute to cancer initiation and progression.[8]
Characteristics
RIOK 1 has a molecular weight of 65,583 Da, a basal isoelectric point of 5.84 (predict pI for various phosphorylation states; pI for unphosphorylated state = 5.84), and a chromosomal location of human orthodox 6p24.3. (6:7,389,496-7,418,037)
PTM Effects
Effects on modified protein - protein degradation, triggered by K411-m1; protein stabilization, triggered by T410-p; ubiquitination, triggered by K411-m1.
Effects on biological progress - cell growth, inhibited, triggered by K411-m1. [9]
Mutagenesis
The effect of the experimental mutation of one or more amino acid(s) on the biological properties of the protein. When amino acid residues are altered, we report the change, the name of the mutant (if known), and the effects of the mutation on the protein, the cell or the complete organism. Examples: Q1LCS4, P04395, Q38914.
When the mutation is associated with several point mutations, we add the exact combination of mutations (positions and amino acid modifications). Examples: P62166, O14776.[10]
The mutation (D324A) in RIOK1 abolishes autophosphorylation activity, enhances association with pre-40S ribosomal subunits and inhibits processing of 18S-E pre-rRNA to the mature 18S rRNA. [11]
Function
Inmunne Repressor
Despite the fact that RIOK1 functions remain unclear, it's been discovered that the lack of this protein grants resistance to a certain type of bacteria called Aeromonas, which shows its function as an inmune repressor.[12]
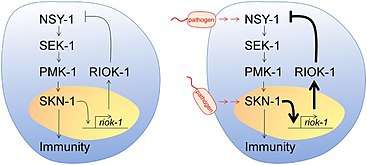
The feedback loop is the model which RIOK1 allows the inhibition of our immune system against bacteria among p38 MAPK and SKN-1. Microorganism presence active the p38 MAPK pathway increasing the concentration of SKN-1, which will end up producing the necessary amount of RIOK1 to stop this pathway.
RNA Maturation
In addition, RIOK1 has also a potential role with the metabolism of the 40S ribosomal subunit, precisely, we know it's involved in the maturation of the 40S ribosomal subunit and needed for the recycling of PNO1 and NOB1, which are both RNA-binding proteins from 40S precursors.[11]
Protein Binding
Furthermore, RIOK1 protein binding function stands out among other proteins involved in the same activity. For instance, in the binding of PRMT5 in which RIOK1 and PICln are involved, suggest that RIOK1 is a more general adapter than PICln. RIOK1 also interacts with NCL via its C-terminus, which targets NCL for PRMT5 methylation.[13].
lists of the major functions and processes of RIOK1:[14]
Functions
- ATP binding
- Hydrolase activity
- Metal ion binding
- Protein binding
- Protein serine/kerotonine kinase activity
Processes it's involved
- Maturation of SSUU-rRNA
- Positive regulation of rRNA processing
- Protein Phosphorylation
- Ribosomal small subunit biogenesis
Location and structure
RIOK1 is the only component of the PRMT5 complex located exclusively in the cytoplasm [13].
Tissue expression
The protein Kinase RIO1 highest expression is in testicles, in addition the RNA that encodes this protein has low tissue especifity, as it is detected in every kind of tissue, but mostly in the pituitary gland, testicles, skeletal muscle, thymus and NK-cells (RIOK1 tissue expression)
Sequence and primary structure
RIOK1 gene has 5 different transcripts [5] but only transcript variant 1 (mRNA) (RIOK1-202) contains an ORF (NCBI GenBank), whose origin sequence is formed of 17 coding exons (represented in red): [5]
RIOK1 transcript variant 1 encodes the protein kinase RIO1 (isoform 1) which contains 568 aminoacids (NCBI GenPept) [15]. As the result of posttranslational modifications the protein Kinase RIO1 has 2 phosphoserines in positions 21 and 22 [16]
Secondary Structure
Its secondary structure consist of 9 alpha helix (red) and 7 beta strands (blue) (Protein Data Bank in Europe ) [17]
Native State
RIOK1 belongs to the serine/threonine-specific protein kinase family and therefore has the protein kinase domain in positions 180-479
Sites
This enzyme has 3 binding sites in positions 208 (for ATP), 278 (for ATP via carbonyl oxygen) and 280 (for ATP via nitrogen amide); 2 metal binding sites [Mg(+2)] in positions 329 and 349 and 2 active sites in positions 324 (which is a proton acceptor) and 341 (4-aspartylphosphate intermediate) [11][17]
References
- GRCh38: Ensembl release 89: ENSG00000124784 - Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000021428 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "GRCh38: Ensembl release 89: ENSG00000124784". May 2017.
- "EC 2.7.11.1". KEGG: Kyoto Encyclopedia of Genes and Genomes.
- "Serine/threonine-protein kinase RIO1". Target Report Card. Hinxton, Cambridgeshire, CB10 1SD, UK: EMBL-EBI, Wellcome Genome Campus. Retrieved 2019-10-24.CS1 maint: location (link)
- Berto G, Ferreira-Cerca S, De Wulf P (April 2019). "The Rio1 protein kinases/ATPases: conserved regulators of growth, division, and genomic stability". Current Genetics. 65 (2): 457–466. doi:10.1007/s00294-018-0912-y. PMID 30515528.
- "RIOK1 (human)". www.phosphosite.org. Retrieved 2019-10-24.
- "Mutagenesis". www.uniprot.org. Retrieved 2019-10-24.
- Barbara, Widmann (2012). "The kinase activity of human Rio1 is required for final steps of cytoplasmic maturation of 40S subunits". Molecular Biology of the Cell. MboC. 23 (1): 22–35. doi:10.1091/mbc.E11-07-0639. PMC 3248900. PMID 22072790.
- CS, Chen (2018). "RIOK-1 Is a Suppressor of the p38 MAPK Innate Immune Pathway in Caenorhabditis elegans". Frontiers in Immunology. 9: 774. doi:10.3389/fimmu.2018.00774. PMC 5913292. PMID 29719537.
- Guderian G, Peter C, Wiesner J, Sickmann A, Schulze-Osthoff K, Fischer U, Grimmler M (January 2011). "RioK1, a new interactor of protein arginine methyltransferase 5 (PRMT5), competes with pICln for binding and modulates PRMT5 complex composition and substrate specificity". The Journal of Biological Chemistry. 286 (3): 1976–86. doi:10.1074/jbc.M110.148486. PMC 3023494. PMID 21081503.
- "RIOK1 RIO kinase 1 [Homo Sapiens (Human)]". RIOK1-NCB1. NCBI. Retrieved 26 October 2019.
- "UniProtKB - Q9BRS2 (RIOK1_HUMAN)".
- Mayya V, Lundgren DH, Hwang SI, Rezaul K, Wu L, Eng JK, Rodionov V, Han DK (Aug 2009). "Quantitative phosphoproteomic analysis of T cell receptor signaling reveals system-wide modulation of protein-protein interactions". Science Signaling. 2 (18): ra46. doi:10.1126/scisignal.2000007. PMID 19690332.
- Ferreira-Cerca S, Kiburu I, Thomson E, LaRonde N, Hurt E (July 2014). "Dominant Rio1 kinase/ATPase catalytic mutant induces trapping of late pre-40S biogenesis factors in 80S-like ribosomes". Nucleic Acids Research. 42 (13): 8635–47. doi:10.1093/nar/gku542. PMC 4117770. PMID 24948609.





