RHPN2
Rhophilin-2 is a protein that in humans is encoded by the RHPN2 gene.[5][6]
| RHPN2 | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||
| Identifiers | |||||||||||||||||||||||||
| Aliases | RHPN2, P76RBE, RHOBP, rhophilin, Rho GTPase binding protein 2, rhophilin Rho GTPase binding protein 2 | ||||||||||||||||||||||||
| External IDs | OMIM: 617932 MGI: 1289234 HomoloGene: 12407 GeneCards: RHPN2 | ||||||||||||||||||||||||
| |||||||||||||||||||||||||
| |||||||||||||||||||||||||
| Orthologs | |||||||||||||||||||||||||
| Species | Human | Mouse | |||||||||||||||||||||||
| Entrez | |||||||||||||||||||||||||
| Ensembl | |||||||||||||||||||||||||
| UniProt | |||||||||||||||||||||||||
| RefSeq (mRNA) | |||||||||||||||||||||||||
| RefSeq (protein) | |||||||||||||||||||||||||
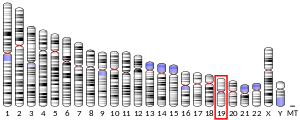
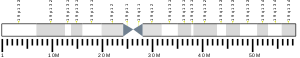
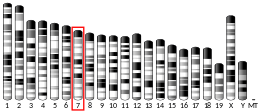
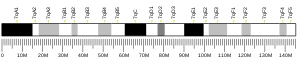
| Location (UCSC) | Chr 19: 32.98 – 33.06 Mb | Chr 7: 35.33 – 35.39 Mb | |||||||||||||||||||||||
| PubMed search | [3] | [4] | |||||||||||||||||||||||
| Wikidata | |||||||||||||||||||||||||
| |||||||||||||||||||||||||
Interactions
RHPN2 has been shown to interact with RHOB.[7]
gollark: * models
gollark: Okay, the auction is consistent with exactly 0 (zero) of my modles.
gollark: μhahahaha. I have the obelisk bid.
gollark: http://mondecitronne.com/main/auction
gollark: I *will* obtain the obelisk even if [BEES EXPUNGED].
References
- GRCh38: Ensembl release 89: ENSG00000131941 - Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000030494 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- Peck JW, Oberst M, Bouker KB, Bowden E, Burbelo PD (November 2002). "The RhoA-binding protein, rhophilin-2, regulates actin cytoskeleton organization". J. Biol. Chem. 277 (46): 43924–32. doi:10.1074/jbc.M203569200. PMID 12221077.
- "Entrez Gene: RHPN2 rhophilin, Rho GTPase binding protein 2".
- Mircescu H, Steuve S, Savonet V, Degraef C, Mellor H, Dumont JE, Maenhaut C, Pirson I (December 2002). "Identification and characterization of a novel activated RhoB binding protein containing a PDZ domain whose expression is specifically modulated in thyroid cells by cAMP". Eur. J. Biochem. 269 (24): 6241–9. doi:10.1046/j.1432-1033.2002.03343.x. PMID 12473120.
Further reading
- Mircescu H, Steuve S, Savonet V, Degraef C, Mellor H, Dumont JE, Maenhaut C, Pirson I (2002). "Identification and characterization of a novel activated RhoB binding protein containing a PDZ domain whose expression is specifically modulated in thyroid cells by cAMP". Eur. J. Biochem. 269 (24): 6241–9. doi:10.1046/j.1432-1033.2002.03343.x. PMID 12473120.
- Jaffe AB, Aspenström P, Hall A (2004). "Human CNK1 acts as a scaffold protein, linking Rho and Ras signal transduction pathways". Mol. Cell. Biol. 24 (4): 1736–46. doi:10.1128/MCB.24.4.1736-1746.2004. PMC 344169. PMID 14749388.
- Barrios-Rodiles M, Brown KR, Ozdamar B, Bose R, Liu Z, Donovan RS, Shinjo F, Liu Y, Dembowy J, Taylor IW, Luga V, Przulj N, Robinson M, Suzuki H, Hayashizaki Y, Jurisica I, Wrana JL (2005). "High-throughput mapping of a dynamic signaling network in mammalian cells". Science. 307 (5715): 1621–5. doi:10.1126/science.1105776. PMID 15761153.
- Benzinger A, Muster N, Koch HB, Yates JR, Hermeking H (2005). "Targeted proteomic analysis of 14-3-3 sigma, a p53 effector commonly silenced in cancer". Mol. Cell. Proteomics. 4 (6): 785–95. doi:10.1074/mcp.M500021-MCP200. PMID 15778465.
- Rual JF, Venkatesan K, Hao T, Hirozane-Kishikawa T, Dricot A, Li N, Berriz GF, Gibbons FD, Dreze M, Ayivi-Guedehoussou N, Klitgord N, Simon C, Boxem M, Milstein S, Rosenberg J, Goldberg DS, Zhang LV, Wong SL, Franklin G, Li S, Albala JS, Lim J, Fraughton C, Llamosas E, Cevik S, Bex C, Lamesch P, Sikorski RS, Vandenhaute J, Zoghbi HY, Smolyar A, Bosak S, Sequerra R, Doucette-Stamm L, Cusick ME, Hill DE, Roth FP, Vidal M (2005). "Towards a proteome-scale map of the human protein-protein interaction network". Nature. 437 (7062): 1173–8. doi:10.1038/nature04209. PMID 16189514.
- Zhan X, Desiderio DM (2006). "Nitroproteins from a human pituitary adenoma tissue discovered with a nitrotyrosine affinity column and tandem mass spectrometry". Anal. Biochem. 354 (2): 279–89. doi:10.1016/j.ab.2006.05.024. PMID 16777052.
- Steuve S, Devosse T, Lauwers E, Vanderwinden JM, André B, Courtoy PJ, Pirson I (2006). "Rhophilin-2 is targeted to late-endosomal structures of the vesicular machinery in the presence of activated RhoB". Exp. Cell Res. 312 (20): 3981–9. doi:10.1016/j.yexcr.2006.08.028. PMID 17054945.
- Ewing RM, Chu P, Elisma F, Li H, Taylor P, Climie S, McBroom-Cerajewski L, Robinson MD, O'Connor L, Li M, Taylor R, Dharsee M, Ho Y, Heilbut A, Moore L, Zhang S, Ornatsky O, Bukhman YV, Ethier M, Sheng Y, Vasilescu J, Abu-Farha M, Lambert JP, Duewel HS, Stewart II, Kuehl B, Hogue K, Colwill K, Gladwish K, Muskat B, Kinach R, Adams SL, Moran MF, Morin GB, Topaloglou T, Figeys D (2007). "Large-scale mapping of human protein-protein interactions by mass spectrometry". Mol. Syst. Biol. 3 (1): 89. doi:10.1038/msb4100134. PMC 1847948. PMID 17353931.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.