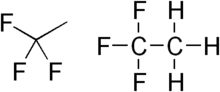
1,1,1-Trifluoroethane
1,1,1-Trifluoroethane, or R-143a or simply trifluoroethane, is a hydrofluorocarbon compound that is a colorless gas. It should not be confused with the much more commonly used gas R-134a or the isomeric compound 1,1,2-trifluoroethane.
 | |
| Names | |
|---|---|
| IUPAC name
1,1,1-Trifluoroethane | |
| Other names
Methylfluoroform, 1,1,1-Trifluoroform, R-143a, HFC-143a, UN 2035 | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.006.361 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C2H3F3 | |
| Molar mass | 84.04 g/mol |
| Appearance | Colourless gas |
| Density | 3.7 kg/m3 (gas) |
| Melting point | −111 °C (−168 °F; 162 K) |
| Boiling point | −47.6 °C (−53.7 °F; 225.6 K) |
| Vapor pressure | 11 200 hPa (20 °C) |
| Hazards | |
EU classification (DSD) (outdated) |
Extremely flammable (F+) |
| R-phrases (outdated) | R12 |
| S-phrases (outdated) | S9, S16, S33 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
It has a critical temperature of 73 °C.
Applications
It is used as a refrigerant either by itself or more commonly as a component of blended mixtures. Unlike CFCs used as refrigerants, trifluoroethane has no chlorine atoms and is therefore is not ozone-depleting. Its high chemical stability and infra-red absorbency make it a potent greenhouse gas with a global warming potential of 4300, higher than many other commonly used HFC refrigerants.[1]
Trifluoroethane is also used as a propellant in canned air products used to clean electronic equipment.
References
- "Refrigerants - Environmental Properties". The Engineering ToolBox. Retrieved 2016-09-12.