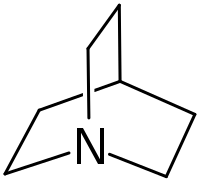
Quinuclidine
Quinuclidine is an organic compound and a bicyclic amine and used as a catalyst and a chemical building block. It is a strong base with pKa of the conjugate acid of 11.0.[3] It can be prepared by reduction of quinuclidone.
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
1-Azabicyclo[2.2.2]octane[2] | |||
| Other names
Quinuclidine[2] | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.002.625 | ||
PubChem CID |
|||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C7H13N | |||
| Molar mass | 111.188 g·mol−1 | ||
| Density | 0.97 g/cm3 | ||
| Melting point | 157 to 160 °C (315 to 320 °F; 430 to 433 K) | ||
| Boiling point | 149.5 °C (301.1 °F; 422.6 K) at 760 mmHg | ||
| Acidity (pKa) | 11.0 (conjugate acid) | ||
| Hazards | |||
| Flash point | 36.5 °C (97.7 °F; 309.6 K) | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
In alkane solvents quinuclidine is a Lewis base that forms adducts with a variety of Lewis acids. It is classified as a soft base and its donor properties are discussed in the ECW model.
The compound is structurally related to DABCO, in which the other bridgehead is also nitrogen, and to tropane, which has a slightly different carbon frame.
Quinuclidine is found as a structural component of some biomolecules including quinine.
References
- Quinuclidine Archived October 15, 2007, at the Wayback Machine at Sigma-Aldrich
- Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 169. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
The name quinuclidine is retained for general nomenclature only (see Table 2.6).
- Hext, N. M.; Hansen, J.; Blake, A. J.; Hibbs, D. E.; Hursthouse, M. B.; Shishkin, O. V.; Mascal, M. (1998). "Azatriquinanes: Synthesis, Structure, and Reactivity". J. Org. Chem. 63 (17): 6016–6020. doi:10.1021/jo980788s. PMID 11672206.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.