Quinoxaline
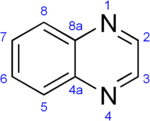
A quinoxaline, also called a benzopyrazine, in organic chemistry, is a heterocyclic compound containing a ring complex made up of a benzene ring and a pyrazine ring. It is isomeric with other naphthyridines including quinazoline, phthalazine and cinnoline.[2] It is a colorless oil that melts just above room temperature. Although quinoxaline itself is mainly of academic interest, quinoxaline derivatives are used as dyes, pharmaceuticals, and antibiotics such as olaquindox, carbadox, echinomycin, levomycin and actinoleutin.
 | |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Quinoxaline | |||
| Other names
Benzo[a]pyrazine, Benzopyrazine, Benzoparadiazine, 1,4-Benzodiazine, Phenopiazine, Phenpiazine, Quinazine, Chinoxalin | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.001.862 | ||
| KEGG | |||
PubChem CID |
|||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C8H6N2 | |||
| Molar mass | 130.150 g·mol−1 | ||
| Melting point | 29-32 °C | ||
| Boiling point | 220 to 223 °C (428 to 433 °F; 493 to 496 K) | ||
| Acidity (pKa) | 0.60[1] | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Synthesis
They can be formed by condensing ortho-diamines with 1,2-diketones. The parent substance of the group, quinoxaline, results when glyoxal is condensed with 1,2-diaminobenzene.[3] Substituted derivatives arise when α-ketonic acids, α-chlorketones, α-aldehyde alcohols and α-ketone alcohols are used in place of diketones.[2] Quinoxaline and its analogues may also be formed by reduction of amino acids substituted 1,5-difluoro-2,4-dinitrobenzene (DFDNB):[4]
 Quinoxaline Synthesis
Quinoxaline Synthesis
Uses
The antitumoral properties of quinoxaline compounds have been of interest.[5] Recently, quinoxaline and its analogs have been investigated as the catalyst's ligands.[6]
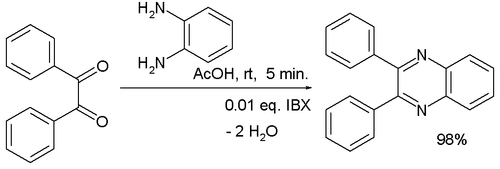
One study used 2-iodoxybenzoic acid (IBX) as a catalyst in the reaction of benzil with 1,2-diaminobenzene:[7]
Pyrazinamide and Morinamide are made out of quinoxaline. It is also used to make amiloride.
References
- Brown, H.C.; et al. (1955). Baude, E.A.; Nachod, F.C. (eds.). Determination of Organic Structures by Physical Methods. New York: Academic Press.
-

- 2,3-Pyrazinedicarboxylic Acid" Reuben G. Jones and Keith C. McLaughlin Org. Synth. 1950, 30, 86. doi:10.15227/orgsyn.030.0086. This paper describes the preparation of quinoxaline as an intermediate.
- Xiang-Hong Wu; Gang Liu; et al. (2004). "Solution-phase reductive cyclization of 2-quinoxalinol analogs: Systematic study of parallel synthesis". Mol. Divers. 8 (2): 165–174. doi:10.1023/B:MODI.0000025639.89179.60.
- Jean Renault; Michel Baron; Patrick Mailliet; et al. (1981). "Heterocyclic quinones.2.Quinoxaline-5,6-(and 5-8)-diones-Potential antitumoral agents". Eur. J. Med. Chem. 16 (6): 545–550.
- Xianghong Wu; Anne E. V. Gorden (2007). "Regioselective Synthesis of Asymmetrically Substituted 2-Quinoxalinol Salen Ligands". J. Org. Chem. 72 (23): 8691–8699. doi:10.1021/jo701395w. PMID 17939720.
- Heravi, Majid M. (2006). "Facile synthesis of quinoxaline derivatives using o-iodoxybenzoic acid (IBX) at room temperature". Arkivoc. 2006 (16). doi:10.3998/ark.5550190.0007.g02.