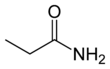
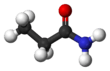
Propanamide
Propanamide has the chemical formula CH3CH2C=O(NH2). It is the amide of propanoic acid.
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Propanamide | |||
| Other names
n-propylamide Propionamide Propylamide Propionic amide | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.001.066 | ||
| EC Number |
| ||
| MeSH | C034666 | ||
PubChem CID |
|||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C3H7NO | |||
| Molar mass | 73.095 g·mol−1 | ||
| Appearance | liquid , yellow | ||
| Density | 22 | ||
| Melting point | 80 °C (176 °F; 353 K) | ||
| Boiling point | 213 °C (415 °F; 486 K) | ||
| very soluble in water | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
This organic compound is a mono-substituted amide. Organic compounds of the amide group can react in many different organic processes to form other useful compounds for synthesis.
Preparation
Propanamide can be prepared by the condensation reaction between urea and propanoic acid
or by the dehydration of ammonium propionate
Reactions
Propanamide being an amide can participate in a Hoffman rearrangement to produce ethylamine gas
gollark: .geese magenta
gollark: .goose magenta
gollark: .geese This goose looks kind of like a newspaper
gollark: .geese imagine geese on the planet mars
gollark: .geese artist's rendering of geese on Mars
References
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.