Propanethiol
Propanethiol is an organic compound with the molecular formula C3H8S. It belongs to the group of thiols. It is a colorless liquid with a strong, offensive odor. It is moderately toxic and is less dense than water and slightly soluble in water. It is used as a feedstock for insecticides.[5] It is highly flammable and it gives off irritating or toxic fumes (or gases) in a fire. Heating it will cause rise in pressure with risk of bursting.[6][7]
 | |
| Names | |
|---|---|
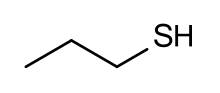
| Preferred IUPAC name
Propane-1-thiol | |
| Other names
n-Propylthiol 1-Propanethiol Propan-1-thiol Propyl mercaptan Mercaptan C3 | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.003.142 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| Properties | |
| C3H8S | |
| Molar mass | 76.16 g·mol−1 |
| Appearance | Colorless to pale yellow liquid |
| Odor | cabbage-like[4] |
| Density | 0.84 g/mL |
| Melting point | −113 °C (−171 °F; 160 K) |
| Boiling point | 67 to 68 °C (153 to 154 °F; 340 to 341 K) |
| Slight[4] | |
| Vapor pressure | 155 mmHg[4] |
| Acidity (pKa) | 8 |
| -58.5·10−6 cm3/mol | |
| Hazards | |
| Flash point | −21 °C; −5 °F; 253 K[4] |
| NIOSH (US health exposure limits): | |
PEL (Permissible) |
none[4] |
REL (Recommended) |
C 0.5 ppm (1.6 mg/m3) [15-minute][4] |
IDLH (Immediate danger) |
N.D.[4] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Chemistry
Propanethiol is chemically classified among the thiols, which are organic compounds with molecular formulas and structural formulas similar to alcohols, except that sulfur-containing sulfhydryl group (-SH) replaces the oxygen-containing hydroxyl group in the molecule. Propanethiol's basic molecular formula is C3H7SH, and its structural formula is similar to that of the alcohol n-propanol.
Propanethiol is manufactured commercially by the reaction of propene with hydrogen sulfide with ultraviolet light initiation in an anti-Markovnikov addition.[8] It can also be prepared by the reaction of sodium hydrosulfide with 1-chloropropane.
References
- International Chemical Safety Card 0317
- ChEBI 8473
- CSID:7560, accessed 19:05, Feb 10, 2013
- NIOSH Pocket Guide to Chemical Hazards. "#0526". National Institute for Occupational Safety and Health (NIOSH).
- 1-Propanethiol Archived 2015-06-20 at the Wayback Machine, chemicalbook.com
- 1-Propanethiol, inchem.org
- 1-Propanethiol, International Chemical Safety Card
- Rector P.Louthan, United States Patent 3,050,452, Aug. 21, 1962, Preparation of Organic Sulfur Compounds