Potassium tetraphenylborate
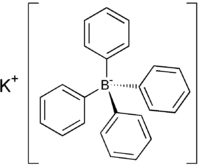
Potassium tetraphenylborate is the salt with the formula KB(C6H5)4). It is a colourless salt that is a rare example of a water-insoluble salt of potassium.
 | |
| Names | |
|---|---|
| IUPAC name
Potassium tetraphenylboranuide | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.156.375 |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C24H20BK | |
| Molar mass | 358.3249 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
The salt has a low solubility in water of only 1.8×10−4 g/L. It is, however, soluble in organic solvents. The insolubility of this compound has been used to determine the concentration of potassium ions by precipitation and gravimetric analysis:[1]
- K+ + NaB(Ph)4 → KB(Ph)4 + Na+
The compound adopts a polymeric structure with bonds between the phenyl rings and potassium. As such it is classified as an organopotassium compound.[2]
References
- Engelbrecht, R. M.; McCoy, F. A. (1956). "Determination of Potassium by Tetraphenylborate Method". Anal. Chem. 28 (11): 1772. doi:10.1021/ac60119a040.
- Ulrich Behrens, Frank Hoffmann, and Falk Olbrich "Solid-State Structures of Base-Free Lithium and Sodium Tetraphenylborates at Room and Low Temperature: Comparison with the Higher Homologues MB(C6H5)4 (M = K, Rb, Cs)" Organometallics 2012, volume 31, p. 905−913. doi:10.1021/om200943n
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.