Potassium ethyl xanthate
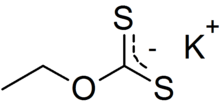
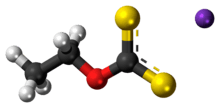
Potassium ethyl xanthate (KEX) is an organosulfur compound with the chemical formula CH3CH2OCS2K. It is a pale yellow powder that is used in the mining industry for the separation of ores. Unlike the related sodium ethyl xanthate, the potassium salt exists as an anhydrous salt.
 | |
 | |
| Names | |
|---|---|
| IUPAC name
potassium O-ethylcarbonodithioate | |
| Other names
potassium ethylxanthogenate potassium-O-ethyl dithiocarbonate | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.004.946 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C3H5KOS2 | |
| Molar mass | 160.29 g·mol−1 |
| Appearance | Pale yellow powder |
| Density | 1.263 g/cm3[1] |
| Melting point | 225 to 226 °C (437 to 439 °F; 498 to 499 K) |
| Boiling point | decomposes |
| Acidity (pKa) | approximately 1.6 |
| Hazards | |
| R-phrases (outdated) | R15 R21 R22 R29 R36 R38 |
| S-phrases (outdated) | S3 S9 S35 S36 S37 S38 S39 S16 S23 S51 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Production and properties
Xanthate salts are prepared by the action of alkoxides on carbon disulfide. The alkoxide is often generated in situ from potassium hydroxide:[2]
- CH3CH2OH + CS2 + KOH → CH3CH2OCS2K + H2O
Potassium ethyl xanthate is a pale yellow powder that is stable at high pH but rapidly hydrolyses at pH <9 at 25 °C. Unlike the sodium derivative, potassium xanthate crystallizes as the anhydrous salt and is non-hygroscopic.
Applications
Potassium ethyl xanthate is used in the mining industry as flotation agent for extraction of the ores of copper, nickel, and silver.[3] The method exploits the affinity of these "soft" metals for the organosulfur ligand.
Potassium xanthate is a useful reagent for preparing xanthate esters from alkyl and aryl halides. The resulting xanthate esters are useful intermediates in organic synthesis.[4]
Safety
The [[]] is 683 mg/kg (oral, rats) for potassium ethyl xanthate.[3]
References
- Report 5 (1995) p. 5
- This report gives a detailed procedure Charles C. Price and Gardner W. Stacy (1948). "p-nitrophenyl) sulfide". Organic Syntheses. 28: 82.; Collective Volume, 3, p. 667
- Kathrin-Maria Roy (2005). "Xanthates". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a28_423.
- One of several procedures using xanthate esters: Fabien Gagosz and Samir Z. Zard (1948). "A Xanthate-Transfer Approach to α-Trifluoromethylamines". Organic Syntheses. 84: 32.; Collective Volume, 11, p. 212