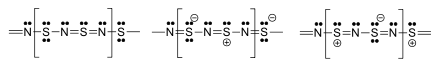Polythiazyl
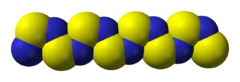
Polythiazyl (polymeric sulfur nitride), (SN)x, is an electrically conductive, gold- or bronze-colored polymer with metallic luster. It was the first conductive inorganic polymer discovered[1][2] and was also found to be a superconductor at very low temperatures (below 0.26 K).[3][4] It is a fibrous solid, described as "lustrous golden on the faces and dark blue-black", depending on the orientation of the sample. It is air stable and insoluble in all solvents.[5]
 | |
x.png) | |
 | |
| Names | |
|---|---|
| Other names
polythiazyl poly(sulfur nitride) | |
| Identifiers | |
| ChemSpider |
|
| Properties | |
| (SN)x | |
| Appearance | bronze colour, metallic lustre[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
History
The compound was first reported as early as 1910 by F.P. Burt, who obtained it by heating tetrasulfur tetranitride in vacuum over silver wool.[6]
The compound was the first non-metallic compound in which superconductivity could be demonstrated. However, the relatively low transition temperature at about 0.3 K makes a practical application unlikely.[7][8]
Properties
Polythiazyl is a metallic-golden and shiny, crystalline but fibrous material.[8] The polymer is mostly inert to oxygen and water, but decomposes in air to a grey powder.[9][10] At temperatures above 240 °C explosive decomposition can occur.[11] The compound also explodes on impact.[10]
Polythiazyl shows an anisotropic electrical conductivity. Along the fibres or SN chains, the bond is electrically conductive, perpendicular to it acts as an insulator. The one-dimensional conductivity is based on the bonding conditions in the S-N chain, where each sulfur atom provides two π electrons and each nitrogen atom provides one π electron to form two-center 3π electron bonding units.[8]
Two polymorphic crystal forms were observed in the compound. The monoclinic form I obtained from the synthesis can be converted into an orthorhombic form II by mechanical treatment such as grinding.[12]
Structure and bonding
The material is a polymer. The S and N atoms on adjacent chains align.[2][13][14] Several resonance structures can be written.[15]
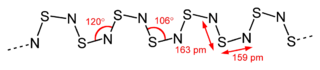
The structure of the crystalline compound was resolved by X-ray diffraction. This showed alternating SN bond lengths of 159 pm and 163 pm and SNS bond angles of 120 °C and NSN bond angles of 106 °C.[16][17][9][8]
Synthesis
Polythiazyl is synthesized by the polymerization of the dimer disulfur dinitride (S2N2), which is in turn synthesized from the cyclic alternating tetramer tetrasulfur tetranitride (S4N4).[2] Conversion from cyclic tetramer to dimer is catalysed with hot silver wool.[2][1][18]
- S4N4 + 8 Ag → 4 Ag2S + 2 N2
- S4N4 (w/ Ag2S catalyst) → 2 S2N2 (w/ 77K cold finger) → S2N2
- S2N2 (@ 0°C, sublimes to surface) → thermal polymerization → (SN)x
Uses
Due to its electrical conductivity, polythiazyl is used in LEDs, transistors, battery cathodes, and solar cells.[18]
Literature
King, R.S.P.: Novel chemistry and applications of polythiazyl, Doctoral Thesis Loughborough University 2009, pdf-Download
References
- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. pp. 725–727. ISBN 978-0-08-037941-8.
- Goehring, Margot; Voigt, Dietrich (1953). "Über die Schwefelnitride (SN)2 und (SN)x". Die Naturwissenschaften (in German). 40 (18): 482. doi:10.1007/BF00628990. ISSN 0028-1042.
- Labes, M. M.; Love, P.; Nichols, L. F. (1979). "Polysulfur Nitride - a Metallic, Superconducting Polymer". Chemical Reviews. 79 (1): 1–15. doi:10.1021/cr60317a002.
- Harry R. Allcock (20 September 2011). Introduction to Materials Chemistry. John Wiley & Sons. p. 131. ISBN 978-1-118-21098-7. Retrieved 29 June 2012.
- A. G. MacDiarmid; C. M. Mikulsk; A. J. Heeger; A. F. Garito (1983). "Polymeric Sulfur Nitride (Polythiazyl), (SN)x". Inorganic Syntheses. 22: 143. doi:10.1002/9780470132531.ch31.
- Burt, Frank Playfair (1910). "XCIX.—A new sulphide of nitrogen" (PDF). J. Chem. Soc., Trans. 97: 1171–1174. doi:10.1039/CT9109701171. ISSN 0368-1645.
- Labes, M.M.; Love, P.; Nichols, L.F.: Polysulfur nitride - a metallic, superconducting polymer in Chem. Rev. 79 (1979) 1–15, doi:10.1021/cr60317a002.
- Alsfasser, R.; Janiak, C.; Klapötke, T.M.; Meyer, H.-J.: Moderne Anorganische Chemie, Herausgeber Riedel, E., 3. Auflage 2007, Walter de Gruyter GmbH & Co. KG, Berlin/Boston, ISBN 978-3-11-019060-1, S. 129–132, (abgerufen über De Gruyter Online).
- MacDiarmid, A.G.; Mikulski, C.M.; Saran, M.S.; Russo, P.J.; Cohen, M.J.; Bright, A.A.; Garito, A.F.; Heeger, A.J.: Synthesis and Selected Properties of Polymeric Sulfur Nitride, (Polythiazyl), (SN)x in Advances in Chemistry 150 (2009) 63–72, doi:10.1021/ba-1976-0150.ch006.
- Entry on Schwefel-Stickstoff-Verbindungen. at: Römpp Online. Georg Thieme Verlag, retrieved 2. März 2017.
- Wiberg, E.; Wiberg, N.; Holleman, A.F.: Anorganische Chemie, 103. Auflage, 2017 Walter de Gruyter GmbH & Co. KG, Berlin/Boston, ISBN 978-3-11-026932-1, S. 681, (abgerufen über De Gruyter Online).
- Baughman, R.H.; Apgar, P.A.; Chance, R.R.; MacDiarmid, A.G.; Garito, A.F.: A New Phase of (SN)x in J. Chem. Soc. Chem. Comm. 1977, 49–50, doi:10.1039/C39770000049.
- Goehring, Margot (1956). "Sulphur nitride and its derivatives". Quarterly Reviews, Chemical Society. 10 (4): 437. doi:10.1039/qr9561000437. ISSN 0009-2681.
- Cohen, M.J .; Garito, A. F.; Heeger, A. J.; MacDiarmid, A. G.; Mikulski, C. M.; Saran, M. S.; Kleppinger, J. (1976). "Solid state polymerization of S2N2 to (SN)x". Journal of the American Chemical Society. 98: 3844–3848. doi:10.1021/ja00429a018.
- Okada, M.; Tanaka, K.; Takata, A.; Yamabe, T. (1993). "Examination of Electronic Phase of the Hartree-Fock Solution of an Isolated Polythiazyl Chain". Synthetic Metals. 59 (2): 223–230. doi:10.1016/0379-6779(93)91029-2.
- Boudeulle, M.: in Cryst. Struct. Comm. 4 (1975) 9–13.
- MacDiarmid, A.G.; Mikulski, C.M.; Russo, P.J.; Saran, M.S.; Garito, A.F.; Heeger, A.J.: Synthesis and structure of the polymeric metal, (SN)x, and its precursor, S2N2 in J. Chem. Soc. Chem. Comm. 1975, 476–477, doi:10.1039/C39750000476.
- Ronald D. Archer (26 February 2001). Inorganic and Organometallic Polymers. John Wiley & Sons. p. 213. ISBN 978-0-471-24187-4. Retrieved 29 June 2012.