Polyprenol
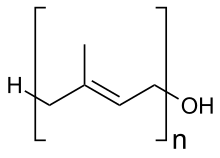
Polyprenols are natural long-chain isoprenoid alcohols of the general formula H-(C5H8)n-OH where n is the number of isoprene units. Any prenol with more than 4 isoprene units is a polyprenol. Polyprenols play an important function acting as natural bioregulators and are found in small quantities in various plant tissues. Dolichols, which are found in all living creatures, including humans, are their 2,3-dihydro derivatives.[1]
 | |
| Identifiers | |
|---|---|
| ChemSpider |
|
| Properties | |
| H-(C5H8)n-OH | |
| Appearance | Transparent oily liquid |
| Density | 0.902–0.905 g/cm3 |
| Insoluble | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Sources
Live trees are known to contain polyprenols. The needles of conifer trees are one of the richest sources of polyprenols.[2] They are also present in shiitake mushrooms in trace amounts.[3]
Research
Polyprenols have been studied for more than 30 years. Interest has been strongest in Russia, Europe, Japan, India, and the United States. In the early 1930s, a scientific team at the Forest Technical Academy in St Petersburg, Russia led by Fyodor Solodky, the founder of Forest Biochemistry, and Asney Agranet, began research into the composition of conifer tree needles. They were intrigued by the trees' ability to remain disease free in extremes of temperature ± 40 °C. Development of Solodky's research led Russian scientists to isolate a completely different class of organic substance from the needles, including polyprenols.
Functions
Polyprenols are low molecular natural bioregulators (physiologically active), playing a significant modulating role in the cellular process in plants referred to as biosynthesis.
What polyprenols are to plants, dolichols are to all living creatures, including man. They are in fact of a very similar chemical composition. Dolichols are a derivative of polyprenols with a saturated isoprene unit.
Through dolichols, the dolichol phosphate cycle occurs. The dolichol phosphate cycle plays a major role in the synthesis of glycoproteins.
All proteins from secretions, membranes and intracellular glycoproteins form the basis for the building of membrane receptors which are used in the production of insulin, adrenaline, estrogen, testosterone and other hormones and enzymes. Seemingly, dolichols have an important role in maintenance of the correct lipid composition of membranes. Decreased levels of dolichols are registered with acute rheumatism and other immunodeficient conditions.
The dolichol phosphate cycle facilitates the process of cellular membrane glycosylation, that is, the synthesis of glycoproteins that control the interactions of cells, support the immune system and the stabilization of protein molecules. Out of all these glycoproteins, polyglycoprotein has the capacity to kill cancerous cells during chemotherapy whilst protecting healthy cells within the body.
The pharmacological activity of polyprenols takes place in the liver where they are metabolized into dolichols.[4]
Potential medical applications
The interest in polyprenols and dolichols is associated with their wide range of demonstrated biological activity and extremely low toxicity.
Polyprenols stimulate the immune system, cellular reparation and spermatogenesis, and have antistress, adaptogenic, antiulcerogenic and wound-healing activity.[5] Dolichols have antioxidant activity and protect cell membranes from peroxidation.[6] Experiments on mice have demonstrated that polyprenols have antiviral activity, in particular against influenza viruses.[7] It has been established that the dolichol level in liver tumor cells are reduced in comparison with healthy hepatic cells.[8]
The Australian pharmaceutical company Solagran Limited has been investigating the medical significance of polyprenols.[9][10]
References
- Rezanka T, Vortuba J (2001). "Chromatography of long chain alcohols (polyprenols) from animal and plant sources". J. Chromatogr. A. 936 (1–2): 95–110. doi:10.1016/S0021-9673(01)01152-9. PMID 11761009.
- Kazimierczak B.; Hertel J.; Swiezewska E.; et al. (1997). "On the specific pattern of long chain polyprenols in green needles of Pinus mugo Turra". Acta Biochim. Pol. 44 (4): 803–808. PMID 9584863.
- Wojtas, M; Bieñkowski, T; Tateyama, S; Sagami, H; Chojnacki, T; Danikiewicz, W; Swiezewska, E (2004). "Polyisoprenoid alcohols from the mushroom Lentinus edodes". Chemistry and Physics of Lipids. 130 (2): 109–115. doi:10.1016/j.chemphyslip.2004.02.007. PMID 15172827.
- Chojnacki T.; Dallner G.J. (1983). "The uptake of dietary polyprenols and their modification to active dolichols by the rat liver". J. Biol. Chem. 258 (2): 916–922. PMID 6401722.
- Roschin V.I. Chemical composition of lipid fraction of green pine and spruce needles. In edition Study and application of therapeutic-prophylactic medications based on natural biologically active compounds. Edited by V.G. Bespalov and V.B. Nekrasova, SPb. Eskulap, 2000, pp.114-116
- Bergamini E.; Bizzarri R.; Cavallini G.; et al. (2004). "Ageing and oxidative stress: a role for dolichol in the antioxidant machinery of cell membranes?". J. Alzheimer's Dis. 6: 129–135.
- Safatov A.S.; Boldyrev A.N.; Bulychev L.E.; et al. (2005). "A prototype prophylactic anti-influenza preparation in aerosol form on the basis of Abies sibirica polyprenols". J. Aerosol. Med. 18 (1): 55–62. doi:10.1089/jam.2005.18.55. PMID 15741774.
- Eggens I.; Elmberger P.G. (1990). "Studies of the polyisoprenoid composition in hepatocellular carcinomas and its correlation with their differentiation". APMIS. 98 (6): 535–542. doi:10.1111/j.1699-0463.1990.tb01068.x. PMID 2166541.
- "Company Announcement" (PDF) (Press release). Solagram Limited. 20 September 2005.
- "Company Announcement" (PDF) (Press release). Solagram Limited. 21 February 2007.