Polyiodide
The polyiodides are a class of polyhalogen anions composed entirely of iodine atoms.[1] The most common and simplest member is the triiodide ion, I−
3. Other known, larger polyiodides include [I4]2−, [I5]−, [I6]2−, [I7]−, [I8]2−, [I9]−, [I10]2−, [I10]4−, [I11]3−, [I12]2−, [I13]3−, [I14]4-, [I16]2−, [I22]4−, [I26]3−, [I26]4−, [I28]4− and [I29]3−.
Preparation
The polyiodides can be made by addition of stoichiometric amounts of I2 to solutions containing I− and I−
3, with the presence of large counter-cations to stabilize them. For example, KI3·H2O can be crystallized from a saturated solution of KI when a stoichiometric amount of I2 is added and cooled.[2]
Structure
3-_ion_in_(((16)aneS4)PdIPd((16)aneS4))(I11).png)
]
4-_in_(DMFc)4(I26).png)
]
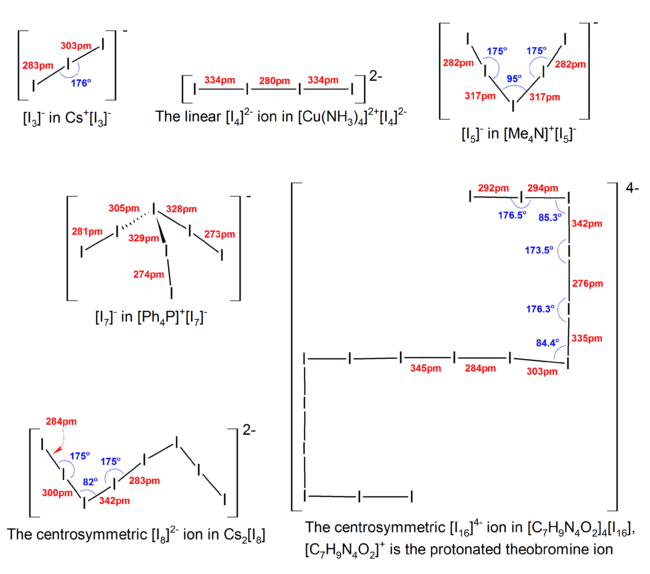
Polyiodides are characterized by their highly complex and variable structures, and can be considered as associations of I2, I−, and I−
3 units. Discrete polyiodides are usually linear, reflecting the origin of the ion. The more complex two- or three-dimensional network structures of chains and cages are formed as the ions interact with each other, with their shapes depending on their associated cations quite strongly. The table below lists the polyiodide salts which have been structurally characterized, along with their counter-cation.[3]
| Anion | Counter-cation | Structural description |
|---|---|---|
| [I3]− | Cs+ | linear |
| [I4]2− | [Cu(NH3)4]2+ | symmetric linear array of iodine atoms[4] |
| [I5]− | [EtMe3N]+ | V-shaped with polymeric layers |
| [EtMePh2N]+ | V-shaped with isolated [I5]− ions | |
| [I6]2- | [NH3-(CH2)8-NH3]2+ | almost linear [[5]] |
| [I7]− | [Ag(18aneS6)]+ | an anionic network derived from a primitive rhombohedral lattice of iodide ions bridged by I2 molecules |
| [I8]2− | [Ni(phen)3]2+ | regular anionic shapes, can be described as [I− 3·I2·I− 8] or [I− 3·I− 5] |
| [I9]− | [Me2iPrPhN]+ | 14-membered ring tied by two I2 bridges to give 10-membered rings |
| [Me4N]+ | non-octahedral, but a twisted "h"-like arrangement of I− 3 and I2 units | |
| [I10]2− | [Cd(12-crown-4)2]2+; Theophyllinium | twisted ring configuration with two I− 3 units linked by two I2 molecules[6] |
| [I11]3− | [(16aneS4)PdIPd(16aneS4)]3+ | 14-membered ring (9.66 × 12.64 Å) around the complex cation, with the rings interlink further to give an infinite 2D sheet |
| [I12]2− | [Ag2(15aneS5)2]2+ | extended 3D spiral superstructure supported by Ag–I bonds and weak I···S interactions |
| [Cu(Dafone)3]2+ | planar configuration | |
| [I13]3− | [Me2Ph2N]+ | consists of zigzag chains of I− and I2 |
| [I14]4- | 4,4'-bipyridinium | double hook (I3- . I2 . I- . I2 . I- . I2 . I3−)[7] |
| [I16]2− | [Me2Ph2N]+ | centrosymmetric arrangement of [I7−·I2·I7−] |
| [iPrMe2PhN]+ | the anion forms 14-membered rings catenated by I2 molecules, which further link into layers with 10- and 14-membered rings | |
| [I22]4− | [MePh3P]+ | two "L"-shaped [I5]− units linked by an I2 molecule and completed by two end-on [I5]− groups |
| [I26]3− | [Me3S]+ | consists of [I5]− and [I7]− ions with intercalated I2 molecules |
| [I26]4− | Cp*2Fe+ | an anionic network derived from a primitive cubic lattice built from I− ions, with I2 bridges on all edges and systematically removing 1⁄12 of the I2 molecules |
| [I29]3− | Cp2Fe+ | an anionic 3D network with a cage-like structure of [{(I− 5)1⁄2·I2}·{(I2− 12)1⁄2·I2}·I2], with [Cp2Fe]+ ions interacting with the anion in the cavities[8] |
| [I∞]δ− | Pyrroloperylene+• | Infinite polyiodide homopolymer.[9] |
References
- Housecroft, Catherine E.; Sharpe, Alan G. (2008). "Chapter 17: The group 17 elements". Inorganic Chemistry (3rd ed.). Pearson. p. 547. ISBN 978-0-13-175553-6.
- Brauer, G., ed. (1963). "Potassium triiodide". Handbook of Preparative Inorganic Chemistry. 1 (2nd ed.). New York: Academic Press. p. 294.
- King, R. Bruce (2005). "Chlorine, Bromine, Iodine, & Astatine: Inorganic Chemistry". Encyclopedia of Inorganic Chemistry (2nd ed.). Wiley. p. 747. ISBN 9780470862100.
- Svensson, Per H.; Kloo, Lars (2003). "Synthesis, Structure, and Bonding in Polyiodide and Metal Iodide–Iodine Systems". Chem. Rev. 103 (5): 1649–84. doi:10.1021/cr0204101. PMID 12744691.
- Reiss, Guido J.; Van Megen, Martin (2013). "I~6~^2−^ Anion Composed of Two Asymmetric Triiodide Moieties: A Competition between Halogen and Hydrogen Bond". Inorganics. 1 (1): 3–13. doi:10.3390/inorganics1010003.
- Reiss, Guido J. (2019-06-26). "A cyclic I102− anion in the layered crystal structure of theophyllinium pentaiodide, C7H9I5N4O2". Zeitschrift für Kristallographie - New Crystal Structures. 234 (4): 737–739. doi:10.1515/ncrs-2019-0082. ISSN 2197-4578.
- Reiss, Guido J.; Megen, Martin van (2012). "Two New Polyiodides in the 4,4'-Bipyridinium Diiodide/Iodine System". Zeitschrift für Naturforschung B. 67 (1): 5–10. doi:10.1515/znb-2012-0102. ISSN 1865-7117.
- Tebbe, Karl-Friedrich; Buchem, Rita (1997-06-16). "Das bisher iodreichste Polyiodid: Herstellung und Struktur von Fc3I29". Angewandte Chemie (in German). 109 (12): 1403–1405. doi:10.1002/ange.19971091233.
- Madhu, Sheri; Evans, Hayden A.; Doan-Nguyen, Vicky V. T.; Labram, John G.; Wu, Guang; Chabinyc, Michael L.; Seshadri, Ram; Wudl, Fred (4 July 2016). "Infinite Polyiodide Chains in the Pyrroloperylene-Iodine Complex: Insights into the Starch-Iodine and Perylene-Iodine Complexes". Angewandte Chemie International Edition. 55 (28): 8032–8035. doi:10.1002/anie.201601585. PMID 27239781.