Point of zero charge
The point of zero charge (pzc) is generally described as the pH at which the net charge of total particle surface (i.e. absorbent's surface) is equal to zero, which concept has been introduced in the studies dealt with colloidal flocculation to explain pH affecting the phenomenon.[1]
A related concept in electrochemistry is the electrode potential at the point of zero charge. Generally, the pzc in electrochemistry is the value of the negative decimal logarithm of the activity of the potential-determining ion in the bulk fluid.[2] The pzc is of fundamental importance in surface science. For example, in the field of environmental science, it determines how easily a substrate is able to adsorb potentially harmful ions. It also has countless applications in technology of colloids, e.g., flotation of minerals. Therefore, the pzc value has been examined in many application of adsorption to the environmental science.[3][4] The pzc value is typically obtained by titrations and several titration method has been developed.[5][6] Related values associated with the soil characteristics exist along with the pzc value, including zero point of charge (zpc), point of zero net charge (pznc), etc.[7]
Term definition of point of zero charge
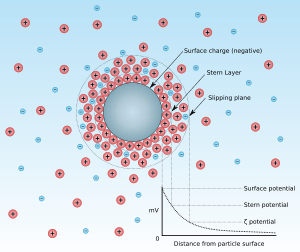
The point of zero charge is the pH for which the net surface charge of adsorbent is equal to zero. This concept has been introduced by an increase of interest in the pH of the solution during adsorption.[1] The reason why pH has attracted much attention is that the adsorption of some substances is very dependent on pH. The pzc value is determined by the characteristics of an adsorbent. For example, the surface charge of adsorbent is described by the ion that lies on the surface of the particle (adsorbent) structure like image. At the lower pH, hydrogen ions (protons, H+) would be adsorbed more than other cations (adsorbate) so that the other cations would be less adsorbed in the case of the negatively charged particle. On the other hand, if the surface is positively charged and pH is increased, anions will be less adsorbed as hydroxide ions are increased. From the view of the adsorbent, if the pH is below the pzc value, the surface charge of adsorbent would be positive so that the anions can be adsorbed. Conversely, if the pH is above the pzc value, the surface charge would be negative so that the cations can be adsorbed.
For example, the charge on the surface of silver iodide crystals may be determined by the concentration of iodide ions in the solution above the crystals. Then, the pzc value of the AgI surface will be described by the concentration of I− in the solution (or negative decimal logarithm of this concentration, pI−).
Relation of pzc to isoelectric point
The pzc is the same as the isoelectric point (iep) if there is no adsorption of other ions than the potential determining H+/OH− at the surface.[8] This is often the case for pure ("pristine surface") oxides in water. In the presence of specific adsorption, pzc and isoelectric point generally have different values.
Method of experimental determination
The pzc is typically obtained by acid-base titrations of colloidal dispersions while monitoring the electrophoretic mobility of the particles and the pH of the suspension. Several titrations are required to distinguish pzc from iep, using different electrolytes (including varying the electrolyte ionic strength). Once satisfactory graphs are obtained (acid/base amount—pH, and pH—zeta potential), the pzc is established as the common intersection point (cip) of the lines. Therefore, pzc is also sometimes referred to as cip.
Related abbreviations
Besides pzc, iep, and cip, there are also numerous other terms used in the literature, usually expressed as initialisms, with identical or (confusingly) near-identical meaning: zero point of charge (zpc), point of zero net charge (pznc), point of zero net proton charge (pznpc), pristine point of zero charge (ppzc), point of zero salt effect (pzse), zero point of titration (zpt) of colloidal dispersion, and isoelectric point of the solid (ieps)[9] and point of zero surface tension (pzst[10] or pzs[11]).
Application in electrochemistry
In electrochemistry, the electrode-electrolyte interface is generally charged. If the electrode is polarizable, then its surface charge depends on the electrode potential.
IUPAC defines[2] the potential at the point of zero charge as the potential of an electrode (against a defined reference electrode) at which one of the charges defined is zero.
The potential of zero charge is used for determination of the absolute electrode potential in a given electrolyte.
IUPAC also defines the potential difference with respect to the potential of zero charge as:
- Epzc = E − Eσ=0
where:
- Epzc is the electrode potential difference with respect to the point of zero charge, Eσ=0
- E is the potential of the same electrode against a defined reference electrode in volts
- Eσ=0 is the potential of the same electrode when the surface charge is zero, in the absence of specific adsorption other than that of the solvent, against the reference electrode as used above, in volts
The structure of electrolyte at the electrode surface can also depend on the surface charge, with a change around the pzc potential. For example, on a platinum electrode, water molecules have been reported to be weakly hydrogen-bonded with "oxygen-up" orientation on negatively charged surfaces, and strongly hydrogen-bonded with nearly flat orientation at positively charged surfaces.[12]
At pzc, the colloidal system exhibits zero zeta potential (that is, the particles remain stationary in an electric field), minimum stability (exhibits maximum coagulation or flocculation rate), maximum solubility of the solid phase, maximum viscosity of the dispersion, and other peculiarities.
Application in environmental geochemistry
In the field of environmental science, adsorption is involved in many parts of technologies that can eliminate pollutants and governs the concentration of chemicals in soils and/or atmosphere. When studying pollutant degradation or the geochemical process, the pzc value related to adsorption has been examined. For example, natural and organic substrates including wood ash, sawdust, etc. are to be used as an adsorbent by eliminating harmful heavy metals like arsenic, cobalt, mercury ion and so forth in contaminated neutral drainage (CND), which is a passive reactor that could possible metal adsorption with low-cost materials. Therefore, the pzc values of the organic substrates were evaluated to optimize the selection of materials in CND.[3] Another example is that the emission of nitrous acid, which controls the atmosphere's oxidative capacity. Different soil pH leads to the different surface charges of minerals so the emission of nitrous acid would be varied, further impacting on the biological cycle involved in the nitrous acid species.[4]
Further reading
- Kosmulski M. (2009). Surface Charging and Points of Zero Charge. CRC Press; 1st edition (Hardcover). ISBN 978-1-4200-5188-9
References
- Sposito, Garrison (1998). "On Points of Zero Charge". Environmental Science & Technology. 32 (19): 2815–2819. doi:10.1021/es9802347. ISSN 0013-936X.
- IUPAC Gold Book
- Bakatula, Elisee Nsimba; Richard, Dominique; Neculita, Carmen Mihaela; Zagury, Gerald J. (2018). "Determination of point of zero charge of natural organic materials". Environmental Science and Pollution Research. 25 (8): 7823–7833. doi:10.1007/s11356-017-1115-7. ISSN 1614-7499.
- Donaldson, Melissa A.; Bish, David L.; Raff, Jonathan D. (2014). "Soil surface acidity plays a determining role in the atmospheric-terrestrial exchange of nitrous acid". Proceedings of the National Academy of Sciences. 111 (52): 18472–18477. doi:10.1073/pnas.1418545112. ISSN 0027-8424. PMC 4284574. PMID 25512517.
- Nasiruddin Khan, M.; Sarwar, Anila (2007). "Determination of points of zero charge of natural and treated adsorbents". Surface Review and Letters. 14 (3): 461–469. doi:10.1142/S0218625X07009517. ISSN 0218-625X.
- Bakatula, Elisee Nsimba; Richard, Dominique; Neculita, Carmen Mihaela; Zagury, Gerald J. (2018). "Determination of point of zero charge of natural organic materials". Environmental Science and Pollution Research. 25 (8): 7823–7833. doi:10.1007/s11356-017-1115-7. ISSN 0944-1344. PMID 29294236.
- Kosmulski, Marek (2001). "Chemical Properties of Material Surfaces". Surfactant Science. 20011074. doi:10.1201/9780585418049. ISBN 978-0-8247-0560-2. ISSN 2155-6512.
- Sposito, Garrison (1998). "On Points of Zero Charge". Environmental Science & Technology. 32 (19): 2815–2819. doi:10.1021/es9802347. ISSN 0013-936X.
- Marek Kosmulski, "Chemical Properties of Material Surfaces", Marcel Dekker Inc., 2001.
- Jean-Pierre Jolivet, "Metal Oxide Chemistry and Synthesis", John Wiley & Sons, 2000.
- R. J. Stol & P. L. de Bruyn; "Thermodynamic stabilization of colloids"; Journal of Colloid and Interface Science; 1980; 75 (1): pp. 185–198.
- Osawa, Masatoshi; Tsushima, Minoru; Mogami, Hirokazu; Samjeské, Gabor; Yamakata, Akira (2008). "Structure of Water at the Electrified Platinum−Water Interface: A Study by Surface-Enhanced Infrared Absorption Spectroscopy". J. Phys. Chem. C. 112 (11): 4248–4256. doi:10.1021/jp710386g.