Phosphinane
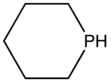
Phosphinane is the organophosphorus compound with the formula (CH2)5PH. This colorless liquid is the parent member of a family of six-membered, saturated rings containing phosphorus. These compounds are mainly of academic interest. The ring adopts a flexible cyclohexane-like chair conformation.[1]
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Phosphinane | |
| Other names
Phosphacyclohexane | |
| Identifiers | |
3D model (JSmol) |
|
PubChem CID |
|
| |
| |
| Properties | |
| C5H11P | |
| Molar mass | 102.117 g·mol−1 |
| Appearance | colorless liquid |
| Boiling point | 118–121 °C (244–250 °F; 391–394 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Phosphinane can be prepared via the Arbuzov reaction of triethylphosphite and 1,5-dibromopentane followed by cyclization and reduction steps.[1] Phosphinane can also be prepared by reduction of 1-chlorophosphinane, which in turn is obtained by the reaction of 1-phenylphosphinane and phosphorus trichloride.[2]
References
- Michael J. Gallagher (2001). "Six-membered rings: Phosphinanes, Dihydro- and Tetrahydro-phosphinines". Phosphorus-Carbon Heterocyclic Chemistry The Rise of a New Domain. pp. 463–483. doi:10.1016/B978-008043952-5/50012-7.
- K. Sommer (1970). "Zur Spaltung tertiärer Phosphine. II". Zeitschrift fuer Anorganische und Allgemeine Chemie. 379: 56–62. doi:10.1002/zaac.19703790110.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.