Phorone
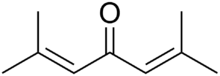
Phorone, or diisopropylidene acetone, is a yellow crystalline substance with a geranium odor, with formula C
9H
14O or ((H
3C–)
2C=CH–)
2C=O.
 | |
| Names | |
|---|---|
| IUPAC name
2,6-Dimethyl-2,5-heptadien-4-one | |
| Other names
Phorone Diisopropylidene acetone | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.007.261 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C9H14O | |
| Molar mass | 138.20 g/mol |
| Density | 0.885 g/cm3 |
| Melting point | 28 °C (82 °F; 301 K) |
| Boiling point | 198 to 199 °C (388 to 390 °F; 471 to 472 K) |
| Hazards | |
| Safety data sheet | External MSDS |
| Flash point | 79 °C (174 °F; 352 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Preparation
It was first obtained in 1837 in impure form by the French chemist Auguste Laurent, who called it "camphoryle".[1] In 1849, the French chemist Charles Frédéric Gerhardt and his student Jean Pierre Liès-Bodart prepared it in a pure state and named it "phorone".[2] On both occasions it was produced by ketonization through the dry distillation of the calcium salt of camphoric acid.[3][4]
It is now typically obtained by the acid-catalysed twofold aldol condensation of three molecules of acetone. Mesityl oxide is obtained as an intermediate and can be isolated.[5]
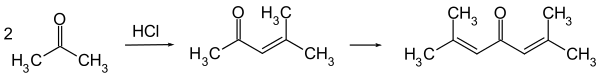
Crude phorone can be purified by repeated recrystallization from ethanol or ether, in which it is soluble.
Reactions
Phorone can condense with ammonia to form triacetone amine.
See also
References
- Merck Index, 11th Edition, 7307.
- Laurent, Auguste (1837). "Sur les acides pinique et sylvique, et sur le camphoryle" [On pinic and sylvic acids, and on camphoryl]. Annales de Chimie et de Physique. 2nd series (in French). 65: 324–332. ; see "Camphoryle", pp. 329–330.
- See:
- Gerhardt, Charles (1849) Comptes rendus des travaux de chimie (Paris, France: Masson, 1849), p. 385. (in French)
- Gerhardt; Liès-Bodart (1849). "Trockne Destillation des camphorsauren Kalks" [Dry distillation of calcium camphorate]. Annalen der Chemie und Pharmacie (in German). 72: 293–294. doi:10.1002/jlac.18490720327. From p. 293: "Dieses Oel, welches Gerhardt und Lies-Bodart mit dem Namen Phoron bezeichnen, … " (This oil, which Gerhardt and Liès-Bodart designate by the name "phorone", … )
- Watts, Henry, A Dicitonary of Chemistry and the Allied Branches of Other Sciences (London, England: Longmans, Green, and Co., 1863), vol. 1, "Camphorone", p. 733.
- Kekulé, August (1866). Lehrbuch der organischen Chemie [Textbook of organic chemistry] (in German). 2nd vol. Erlangen, (Germany): Ferdinand Enke. p. 463.
- Hardo Siegel, Manfred Eggersdorfer (2005). "Ketones". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a15_077.CS1 maint: uses authors parameter (link)