Phenyl-C61-butyric acid methyl ester
PCBM is the common abbreviation for the fullerene derivative [6,6]-phenyl-C61-butyric acid methyl ester. It is being investigated in organic solar cells.[3]
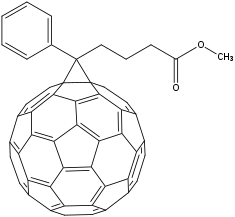 | |
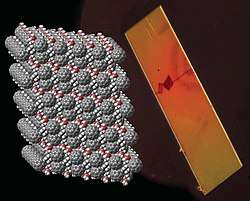 PCBM crystal and its model. Gray: carbons, red: oxygens, white: hydrogens. | |
| Names | |
|---|---|
| Preferred IUPAC name
Phenyl-C61-butyric acid methyl ester | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C72H14O2 | |
| Molar mass | 910.902 g·mol−1 |
| Density | 1.631 g/cm3 (100 K)[1] |
| Melting point | 280 °C (536 °F; 553 K)(sublimates)[2] |
| Structure(100 K)[1] | |
| Monoclinic | |
| P2(1)/n | |
a = 1.347 nm, b = 1.51 nm, c = 1.901 nm α = 90°, β = 106.9°, γ = 90° | |
Formula units (Z) |
4 |
| Hazards | |
| GHS pictograms |  |
| GHS Signal word | Warning |
GHS hazard statements |
H319, H335 |
| P261, P264, P271, P280, P304+340, P305+351+338, P312, P337+313, P403+233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
PCBM is a fullerene derivative of the C60 buckyball that was first synthesized in the 1990s.[4] It is an electron acceptor material and is often used in organic solar cells (plastic solar cells) or flexible electronics in conjunction with electron donor materials such as P3HT or other conductive polymers. It is a more practical choice for an electron acceptor when compared with fullerenes because of its solubility in chlorobenzene. This allows for solution processable donor/acceptor mixes, a necessary property for "printable" solar cells. However, considering the cost of fabricating fullerenes, it is not certain that this derivative can be synthesized on a large scale for commercial applications.
See also
References
| Wikimedia Commons has media related to PCBM. |
- Paternò, Giuseppe; Warren, Anna J.; Spencer, Jacob; Evans, Gwyndaf; Sakai, Victoria García; Blumberger, Jochen; Cacialli, Franco (2013). "Micro-focused X-ray diffraction characterization of high-quality [6,6]-phenyl-C61-butyric acid methyl ester single crystals without solvent impurities" (PDF). Journal of Materials Chemistry C. 1 (36): 5619–5623. doi:10.1039/C3TC31075B.
- Larson, Bryon W.; Whitaker, James B.; Popov, Alexey A.; Kopidakis, Nikos; Rumbles, Garry; Boltalina, Olga V.; Strauss, Steven H. (2014). "Thermal [6,6] → [6,6] Isomerization and Decomposition of PCBM (Phenyl-C61-butyric Acid Methyl Ester)". Chemistry of Materials. 26 (7): 2361–2367. doi:10.1021/cm500594u.
- Björström, Cecilia; Bernasik, Andrzej; Rysz, Jakub; Budkowski, Andrzej; Nilsson, Svante; Svensson, Mattias; Andersson, Mats; Magnusson, Kjell; Moons, Ellen (December 21, 2005). "Multilayer formation in spin-coated thin films of low-bandgap polyfluorene: PCBM blends". Journal of Physics: Condensed Matter. 17 (50): L529–L534. doi:10.1088/0953-8984/17/50/L01.
- Hummelen, Jan C.; Knight, Brian W.; Lepeq, F.; Wudl, Fred; Yao, Jie; Wilkins, Charles L. (1995). "Preparation and Characterization of Fulleroid and Methanofullerene Derivatives". The Journal of Organic Chemistry. 60 (3): 532–538. doi:10.1021/jo00108a012.