Peritoneal carcinomatosis
Peritoneal carcinomatosis (PC) is intraperitoneal dissemination (carcinosis) of any form of cancer that does not originate from the peritoneum itself. PC is most commonly seen in abdominopelvic malignancies. Computed tomography (CT) is particularly important for detailed preoperative assessment and evaluation of the radiological Peritoneal Cancer Index (PCI). The imaging findings vary from simple ascites to multifocal discrete nodules and infiltrative peritoneal masses. Various tumours and tumour like conditions can mimic PC. A systematic analysis of CT imaging features is helpful to narrow down the differential diagnosis, staging and effectively guiding the patient management.[2]
| Peritoneal carcinomatosis | |
|---|---|
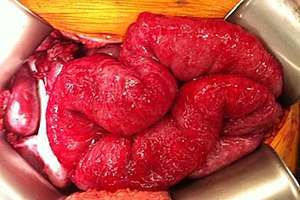 | |
| Intestines with peritoneal carcinomatosis from gastric cancer, appearing as a grainy serosal surface.[1] | |
| Specialty | Oncology |
Introduction
The peritoneum is a mesothelial lining covering the abdominal cavity (parietal peritoneum) and intraperitoneal organs (visceral peritoneum). Peritoneal cavity contains a small amount of fluid, which circulates under the influence of negative pressure generated by the diaphragm, gravity and bowel peristalsis. This natural flow pattern determines the route of spread of disease processes within the peritoneal cavity.[2] Peritoneal carcinomatosis (PC) is defined as intraperitoneal dissemination of any tumor which is not originated from the peritoneum itself. PC is one of the most common diffuse peritoneal diseases.[2]
Radiographic staging
Peritoneal carcinomatosis without distant metastases represents locoregional disease and has the potential for aggressive locoregional treatment. Most CT scan findings are however nonspecific as both neoplastic and non-neoplastic pathologies of the peritoneum present as soft tissue masses, with or without ascites.[2] Computed Tomography provides direct visualization of primary and secondary peritoneal tumor. The detailed information about morphology, size and location of peritoneal implants can be obtained. Multidetector Computed Tomography (MDCT) remains the modality of choice for primary staging, especially in patients with poor compliance for diagnostic examinations, providing a great deal of information about a large volume of tissue and permitting assessment of metastatic extraperitoneal disease. Role is limited in detection of early micronodulation (<5mm implant). MDCT for diagnosis of peritoneal metastasis is highly specific, (specificity ranging from 85- 87%), although sensitivity is low (ranging from 42-47%)[2] A detailed preoperative assessment of PC is essential to provide the surgeon information for evaluation of the radiological Peritoneal Cancer Index (PCI). PCI is considered as an important prognostic indicator, also helpful in guiding therapeutic management.[2] It can also be used in clinical practice during operations.
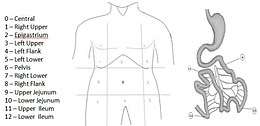
Several methods of classification have been used to investigate the extent of carcinomatosis. The Peritoneal Cancer Index (PCI) of Sugarbaker was chosen by an expert panel as a useful quantitative prognostic tool. It is the most widely validated and precise quantitative prognostic indicator. It was described by Jacquet and Sugarbaker. It assesses the distribution and implant size of the cancer throughout the abdomen and the pelvis quantitatively. The abdomen and pelvis are divided by lines into nine regions. The small bowel is then divided into four regions.[2] (Fig. 1).
| LS 0 | No tumor seen |
| LS 1 | Tumor up to 0.5 cm |
| LS 2 | Tumor up to 5 cm |
| LS 3 | Tumor > 5 cm or confluence |
The lesion size of the largest implant is scored as lesion size 0 through 3 (LS-0 to LS-3). LS-0 means no implants are seen throughout the regions. LS-1 refers to implants that are visible up to 0.5 cm in greatest diameter. LS-2 identifies nodules greater than 0.5 cm and up to 5 cm. LS-3 refers to implants 5 cm or greater in diameter.
Causes
PC is most commonly seen in abdominopelvic malignancies. Ovarian cancer is the commonest cause (46%) followed by colorectal carcinoma (31%), pancreatic cancer, stomach cancer and other malignancies including the hepatocellular carcinoma, gallbladder carcinoma, renal cell carcinoma, transitional cell carcinoma, endometrial, cervical cancers and unknown primary. Extra-abdominal conditions such as breast cancer, lung cancer and malignant melanoma can involve the peritoneal cavity through the haematogenous spread.[2]
Many non-neoplastic and neoplastic conditions may mimic PC, such as tuberculosis, splenosis implant, peritoneal lymphomatosis, pseudomyxoma peritonei and primary peritoneal mesothelioma.[2]
Anatomic considerations
Peritoneum is a thin, translucent serosal membrane of mesodermal origin, covers the surface of the peritoneal cavity, mesenteries and intraperitoneal viscera.[2] Peritoneum is divided into visceral and parietal components. The visceral peritoneum covers the intraperitoneal organs, omenta and mesenteries. The parietal peritoneum lines the abdominal walls; undersurface of the diaphragm; anterior surface of the retroperitoneal viscera and the pelvis.12The peritoneum subdivided into interconnected compartments by the peritoneal ligaments and mesenteries which determine the location and routes of spread of primary and secondary malignancies and infection within the peritoneal cavity. Normally a very small volume of sterile fluid is present in the peritoneal cavity. This fluid continuously circulates upwards to the subdiaphragmatic spaces where the subphrenic submesothelial lymphatics provide most of the lymphatic clearance from the peritoneal cavity. The cephalic movement of peritoneal fluid is augmented by the negative intra-abdominal pressure during exhalation and bowel peristaltic motion.[2]

Initially, peritoneal fluid accumulates in gravity-dependent spaces, the deep recesses of the pelvis and the lateral paravesical spaces and then ascends cephalad through the paracolic gutters reaches the subdiaphragmatic spaces. As the left paracolic gutter is shallow and discontinuous with left subdiaphragmatic space at the phrenicocolic ligament, most of the fluid takes path through the right paracolic gutter. Completion of the circulatory pathway takes place caudally by redirection of fluid into the pelvis through the inframesocolic compartment13 (Fig. 3). In pathologic conditions the excessive fluid collects in well-defined areas of stasis or arrested flow including the peritoneal recesses of the pelvis (pouch of Douglas in women and retrovesical space in men), the right lower quadrant (near the termination of the small bowel mesentery at the ileocaecal junction), the superior aspect of the sigmoid mesocolon and the right paracolic gutter.[2] In the cases of primary and secondary peritoneal malignancies, careful evaluation of the peritoneal recesses is necessary to locate occult disease for proper staging and planning for debulking procedures.[2]
Pathways of spread
Peritoneum can be involved by direct invasion, lymphatic paths, intraperitoneal seeding or hematogenous spread. Direct invasion can be contiguous or non-contiguous. Contiguous peritoneal involvement occurs from the involved organ periphery directly. Non-contiguous direct spread directed by peritoneal reflections, ligaments and lymphatic channels.[2] There are two main routes for lymphatic dissemination including the lymphatic system of the greater omentum and the right side of the subphrenic lymphatic system.[2] Hematogenous route is the main route for primary tumor with a high grade of malignancy. It includes distant metastasis from malignant melanoma, carcinoma breast and lung. Peritoneal seeding is predominantly directed by intraperitoneal circulation of peritoneal fluid.[2]
Common sites of peritoneal implants
Constant circulation of peritoneal fluid allows transcoelomic dissemination of malignant cells. Their deposition and growth occur at particular sites due to relative stasis of ascitic fluid.1 Undersurface of the diaphragm (due to negative pressure and capillary force), omenta (Tendency to move toward the areas of peritoneal irritation), recto-uterine space (pouch of Douglas), right lower quadrant (region of ileocaecal junction), left lower quadrant (superior aspect of sigmoid mesocolon) and right paracolic gutter are prone for metastatic implants.[2]
Spectrum of imaging findings in peritoneal carcinomatosis
Peritoneal carcinomatosis is the most common peritoneal neoplastic condition. It can give wide spectrum of imaging appearances ranging from simple ascites to large peritoneal / omental mass like deposits. Here we present pictorial review of key CT imaging findings with few of the PC mimics.[2]
Ascites
The presence of ascites is one of the first indicators of peritoneal carcinomatosis (Fig. 4). Loculation of ascitic fluid is further a helpful feature. Ascites is seen in up to 70% of PC cases, although it is non-specific. Subphrenic lymphatic obstruction and malignancy associated excess fluid production are the main causing factors for ascites. In some cases, ascites is little or absent (Fig. 5).[2]
Omental involvement
Omentum hangs like an apron from the greater curvature of the stomach and the proximal part of the duodenum, thus it covers the majority of the abdominal organs.[2]
Earlier omental involvement can be manifested by smudge pattern (invasion of fat, with or without small nodules within the fat) (Fig. 6). In later forms, nodular pattern and mixed solid- cystic patterns can be seen (Fig. 7).[2] Subsequently, separation of the colon and the small intestine from the anterior abdominal wall with classic omental cake appearance takes place. (Fig. 8). Ovarian carcinoma is considered to be the most common malignancy producing omental cake.[2]


 Fig. 6. Peritoneal carcinomatosis in 78-year-old female with unknown primary presenting with omental smudge pattern (arrow) and ascites.[2]
Fig. 6. Peritoneal carcinomatosis in 78-year-old female with unknown primary presenting with omental smudge pattern (arrow) and ascites.[2] Fig. 7. Omental deposits in peritoneal carcinomatosis. A. 59-year-old male patient with hepatocellular carcinoma. Ascites with nodular omental deposits (arrowheads). B. Multiple large mass like omental deposits (arrows) with ascites noted in different patient with peritoneal carcinomatosis.[2]
Fig. 7. Omental deposits in peritoneal carcinomatosis. A. 59-year-old male patient with hepatocellular carcinoma. Ascites with nodular omental deposits (arrowheads). B. Multiple large mass like omental deposits (arrows) with ascites noted in different patient with peritoneal carcinomatosis.[2] Fig. 8. Omental cake. Axial CECT image of a 55-year-old female patient with carcinoma stomach showing peritoneal carcinomatosis in the form of omental cake (arrowhead).[2]
Fig. 8. Omental cake. Axial CECT image of a 55-year-old female patient with carcinoma stomach showing peritoneal carcinomatosis in the form of omental cake (arrowhead).[2]
Infiltration of the small bowel mesentery
The mesentery is a double layered peritoneal reflection which suspends the jejunum and the ileum from the posterior abdominal cavity wall. Mesenteric involvement can be manifested by soft-tissue tumor replacement of normal mesenteric fat, in the form of fat stranding, discrete or confluent nodules, mesenteric mass, and anomalous fixation of small bowel loops due to stiff and retractile mesentery, produces characteristic pleated and stellate patterns (Fig. 9, 10).[2] Presence of calcified deposits is pathognomonic for mucin producing tumor cell implants, such as mucinous cystadenocarcinoma ovary (Fig. 11).[2] Small bowel obstruction is one of the most common complications of peritoneal carcinomatosis, can occur secondary to diffusely infiltrating tumor or focal tumor masses.[2]
 Fig. 9. Peritoneal carcinomatosis in 48-year-old male patient of hepatocellular carcinoma with retractile mesentery. Smooth peritoneal thickening (thin arrows) and enhancement with central fixation (thick arrow) of small bowel loops and ascites (stars) noted.[2]
Fig. 9. Peritoneal carcinomatosis in 48-year-old male patient of hepatocellular carcinoma with retractile mesentery. Smooth peritoneal thickening (thin arrows) and enhancement with central fixation (thick arrow) of small bowel loops and ascites (stars) noted.[2] Fig. 10. A. Starry mesentery pattern (arrows) of peritoneal carcinomatosis in a 65-year-old female case of carcinoma pancreas. B. Small bowel mesentery infiltrated with multiple micro and macronodules (arrows) in a 68-year-old female case of peritoneal carcinomatosis with unknown primary.[2]
Fig. 10. A. Starry mesentery pattern (arrows) of peritoneal carcinomatosis in a 65-year-old female case of carcinoma pancreas. B. Small bowel mesentery infiltrated with multiple micro and macronodules (arrows) in a 68-year-old female case of peritoneal carcinomatosis with unknown primary.[2] Fig. 11. A 52-year-old female patient, a follow up case of mucinous cystadenocarcinoma ovary. Mixed solid and cystic peritoneal deposit (white arrow) with calcified mesenteric deposits (curved arrow) and ascites consistent with peritoneal carcinomatosis.[2]
Fig. 11. A 52-year-old female patient, a follow up case of mucinous cystadenocarcinoma ovary. Mixed solid and cystic peritoneal deposit (white arrow) with calcified mesenteric deposits (curved arrow) and ascites consistent with peritoneal carcinomatosis.[2]
Serous Peritoneal implants

- Micronodular: Tiny 1–5 mm milky spots of peritoneal implants diffusely involving the serosa and subserosal fat can be present.
Micronodules are difficult to access with imaging alone.[2]
- Nodular: Nodules with a diameter > 5 mm diffusely involving the serosa and subserosal fat can be seen.[2]
- Plaque like: Confluence of multiple nodular implants forms irregular soft-tissue thickenings, which coat abdominal viscera and
peritoneal walls. It is typically found in subdiaphragmatic spaces (Fig. 12).[2]
- Mass like: Confluence of multiple nodular implants, usually in the pelvis, leads to formation of tissue mass that can reach sizes
of several centimeters (Fig. 13).[2]
Morphological categories of those implants can be either solid, cystic, calcified or mixed (Fig. 14) Ascites, peritoneal thickening and enhancement are commonest findings, followed by omental deposits and caking. PC must be considered as the first possibility in the presence of favorable imaging features even in the absence of detectable primary tumor.[2]
 Fig. 13. A and B. Large discrete and confluent solid mass like deposits involving the omentum and pelvic peritoneum (arrow) in two different cases of peritoneal carcinomatosis.[2]
Fig. 13. A and B. Large discrete and confluent solid mass like deposits involving the omentum and pelvic peritoneum (arrow) in two different cases of peritoneal carcinomatosis.[2] Fig. 14. Morphology of peritoneal deposits patterns in peritoneal carcinomatosis.[2]
Fig. 14. Morphology of peritoneal deposits patterns in peritoneal carcinomatosis.[2]
A. 59-year-old male patient a case of hepatocellular carcinoma with nodular mixed solid and cystic omental deposits (Black arrow).
B. Solid nodular omental and peritoneal deposits (White arrow) in 48-year-old male patient, a case of hepatocellular carcinoma.
C. Cystic peritoneal deposits (Curved arrow), scalloping the liver surface, mimicking liver cysts in case of mucinous cystadenocarcinoma ovary in a 52-year-old female patient.
PC mimics
Tuberculous peritonitis
 Fig. 15. Case of “wet type” of tuberculous peritonitis. A and B. Smudge pattern of greater omental involvement (star) with smooth peritoneal thickening and enhancement. Thickened serosal surface of small and large bowel loops (arrow heads) with ascites.[2]
Fig. 15. Case of “wet type” of tuberculous peritonitis. A and B. Smudge pattern of greater omental involvement (star) with smooth peritoneal thickening and enhancement. Thickened serosal surface of small and large bowel loops (arrow heads) with ascites.[2] Fig. 16. A 24-year-old male patient with fibrotic type of tubercular peritonitis.[2]
Fig. 16. A 24-year-old male patient with fibrotic type of tubercular peritonitis.[2]
A. Marked ascites with centrally displaced bowel loops, mimicking peritoneal carcinomatosis. Splenic microabscesses (curved arrow) also noted.
B. Peripherally enhancing central hypodense mesenteric lymph nodes (arrow).
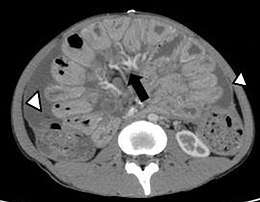
C. Smooth peritoneal thickening and enhancement.[2]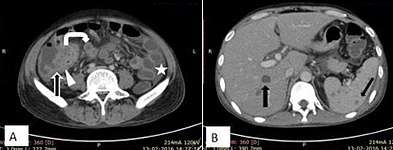 Fig. 17. A 32-year-old male patient, a case of abdominal tuberculosis. A and B. Ileocecal wall thickening (straight arrow), patulous IC junction (arrow head), caseous mesenteric lymphnodes (curved arrow) with ascites (star). B. Hepatic and splenic microabscesses (arrows).[2]
Fig. 17. A 32-year-old male patient, a case of abdominal tuberculosis. A and B. Ileocecal wall thickening (straight arrow), patulous IC junction (arrow head), caseous mesenteric lymphnodes (curved arrow) with ascites (star). B. Hepatic and splenic microabscesses (arrows).[2]
Like tuberculosis, Crohn‟s disease also can result in granulomatous peritonitis (Fig. 18). Smooth and regular peritoneal thickening is more in favor of granulomatous peritonitis.[2]
Splenosis implants
The majority of splenosis implants are found after splenic injury by trauma or after splenectomy. Splenic fragments can become implanted anywhere in the abdominal cavity. With imaging splenosis appears as dense well circumscribed lesion with attenuation and enhancement patterns similar to splenic parenchyma.[2]
Peritoneal lymphomatosis
Diffuse peritoneal involvement can be seen in high grade lymphomas, lymphomas complicating AIDS and Burkitt lymphomas.[2] Diffuse peritoneal thickening with multifocal mass and nodules are characteristic imaging features. Ascites, mesenteric and omental infiltration also can be noted.[2]
Presence of lymph node involvement, encasement of the mesenteric vasculature, producing the „„sandwich‟‟ sign, homogenously enhancing thickened bowel wall with aneurysmal dilatation of the involved segment and splenomegaly are associated findings which are helpful in differentiation (Fig. 19, 20).[2]
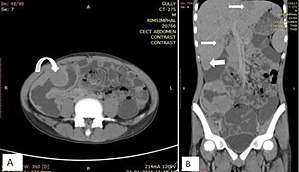 Fig. 19. NHL in a 17 year old HIV positive patient. A. Irregular homogenously enhancing wall thickening involving the ileocaecal region with aneurysmal dilatation of involved segments (curved arrow). B. Hepatosplenomegaly with hepatic metastasis (white arrows).[2]
Fig. 19. NHL in a 17 year old HIV positive patient. A. Irregular homogenously enhancing wall thickening involving the ileocaecal region with aneurysmal dilatation of involved segments (curved arrow). B. Hepatosplenomegaly with hepatic metastasis (white arrows).[2] Fig. 20. Case of gastric lymphoma. A and B. Concentric wall thickening with homogenous contrast enhancement (curved arrows), involving the antrum and pylorus of the stomach without causing gastric outlet obstruction. C. Soft tissue mass encasing the mesenteric vessels without obstruction (white arrow).[2]
Fig. 20. Case of gastric lymphoma. A and B. Concentric wall thickening with homogenous contrast enhancement (curved arrows), involving the antrum and pylorus of the stomach without causing gastric outlet obstruction. C. Soft tissue mass encasing the mesenteric vessels without obstruction (white arrow).[2]
Pseudomyxoma peritonei
Pseudomyxoma peritonei (PMP) is a rare complication of mucinous tumours of appendiceal or ovarian origin that results in peritoneal and omental implants. The CT signs of pseudomyxoma peritonei are not specific, combining peritoneal effusion, peritoneal nodules and invasion of the greater omentum. Gelatinous deposits scalloping over the hepatic margins, loculated ascites and curvilinear calcification are pathognomonic features. The pressure of gelatinous material prevents the bowel loops floating towards the anterior abdominal wall, which may be useful sign in differentiating pseudomyxoma peritonei from ascites.[2]
Malignant peritoneal mesothelioma
Mesothelioma is a rare primary tumour of the connective tissue, originates in the serous membranes of the pleura, peritoneum or pericardium. Peritoneal involvement is reported in 25% of cases.[2] Imaging features include ascites, diffuse irregular nodular peritoneal thickening, invasion of omenta and mesentery with the formation of omental cakes, and mesenteric masses and bowel wall thickening.[2] Coexistence of pleural abnormalities with positive occupational asbestos exposure history in absence detectable primary tumour goes more in favor of mesothelioma. The extent of carcinomatosis represents one of the most important prognostic factors. In patients with PC accurate preoperative assessment is essential to determine a road map for choosing an optimal type of treatment.[2]
Summary
Various primary and secondary peritoneal conditions present with spectrum of imaging features. Analysis of peritoneal involvement patterns are helpful in narrowing down the differential diagnosis. Peritoneal carcinomatosis should be considered if ascites present with irregular peritoneal thickening, nodular omental and peritoneal deposits, omental caking. Attempt should be made to search for primary site. Tuberculosis can present in dry, wet or fibrotic forms and can strongly mimic peritoneal carcinomatosis; ascites with smooth or irregular peritoneal thickening are common findings. Presence of hepatic or splenic miliary microabscesses, splenomegaly, inflammatory thickening of the terminal ileum and caecum and caseous lymphadenopathy in appropriate clinical scenario support abdominal tuberculosis. Pseudomyxoma peritonei should be kept in mind if hypo-attenuating deposits are scalloping the visceral surface of intraperitoneal solid organ margins with calcified peritoneal and/or omental deposits. Presence of nodular peritoneal / omental / mesenteric deposits in scenario of old splenic injury possibility of splenosis should be considered. Lymph node involvement, encasement of the mesenteric vasculature, producing the „„sandwich‟‟ sign are helpful in differentiation of peritoneal lymphomatosis. Knowledge of the peritoneal anatomy together with an understanding of the mechanisms behind malignant tumoural seeding of the peritoneal cavity aids in the interpretation of often complex imaging appearances in peritoneal carcinomatosis. A systematic approach is important for accurate assessment.[2]
References
- Turaga, Kiran K.; Gamblin, T. Clark; Pappas, Sam (2012). "Surgical Treatment of Peritoneal Carcinomatosis from Gastric Cancer". International Journal of Surgical Oncology. 2012: 1–4. doi:10.1155/2012/405652. ISSN 2090-1402.
- Subhaschandra Singh, Y. Sobita Devi, Shweta Bhalothia and Veeraraghavan Gunasekaran (2016). "Peritoneal Carcinomatosis: Pictorial Review of Computed Tomography Findings". International Journal of Advanced Research. 4 (7): 735–748. doi:10.21474/IJAR01/936. ISSN 2320-5407.CS1 maint: multiple names: authors list (link) CC-BY 4.0