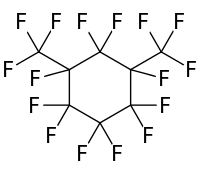
Perfluoro-1,3-dimethylcyclohexane
Perfluoro-1,3-dimethylcyclohexane is a fluorocarbon liquid—a perfluorinated derivative of the hydrocarbon 1,3-dimethylcyclohexane. It is chemically and biologically inert.
 | |
| Names | |
|---|---|
| IUPAC name
1,1,2,2,3,3,4,5,5,6-Decafluoro-4,6-bis(trifluoromethyl)cyclohexane | |
| Other names
Flutec PP3 | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.005.807 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C8F16 | |
| Molar mass | 400.062 g·mol−1 |
| Appearance | Clear, colorless liquid |
| Density | 1.828 g/mL |
| Melting point | −70 °C (−94 °F; 203 K) |
| Boiling point | 102 °C (216 °F; 375 K) |
| 10 ppm | |
| Hazards | |
| Main hazards | None |
| Flash point | None |
| None | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Manufacture
Perfluoro-1,3-dimethylcyclohexane can be manufactured by the Fowler process, which involves moderating the action of elemental fluorine with cobalt fluoride in the gas phase from meta-xylene. This is preferred as the starting material over 1,3-dimethylcyclohexane as less fluorine is required.[1]
Properties
Perfluoro-1,3-dimethylcyclohexane is chemically inert and thermally stable (to over 400 °C). It is non-toxic.[2]
It is a clear, colorless liquid, with a relatively high density, low viscosity and low surface tension that will rapidly evaporate. It is a relatively good solvent for gases, but a poor solvent for solids and liquids.[3]
In common with other cyclic perfluorocarbons, perfluoro-1,3-dimethylcyclohexane can be detected at extremely low concentrations, making it ideal as a tracer.[4]
Applications
- Heat transfer agent
- Dielectric fluid
- Perfluorocarbon tracer
References
- Sandford G (2003). "Perfluoroalkanes". Tetrahedron. 59: 437–454. doi:10.1016/s0040-4020(02)01568-5.
- "FLUTEC PP3". F2 Chemicals.
- "Solubility in Liquids" (PDF). F2 Chemicals.
- Begley P1, Foulger B, Simmonds P. (1988). "Femtogram detection of perfluorocarbon tracers using capillary gas chromatography-electron-capture negative ion chemical ionisation mass spectrometry". J. Chromatogr. 445 (1): 119–128. doi:10.1016/s0021-9673(01)84513-1.CS1 maint: uses authors parameter (link)