Pentanenitrile
Pentanenitrile, valeronitrile or butyl cyanide is a nitrile with the formula C4H9CN. This can be written BuCN, with Bu representing an n-butyl (linear butyl group).
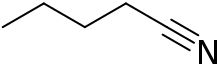 | |
| Names | |
|---|---|
Other names
| |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.003.439 |
| EC Number |
|
PubChem CID |
|
| UNII | |
| UN number | 3273 |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C5H9N | |
| Molar mass | 83.134 g·mol−1 |
| Appearance | Colorless liquid |
| Density | 0.8008 |
| Melting point | −96.2 °C (−141.2 °F; 177.0 K) |
| Boiling point | 141 °C; 286 °F; 414 K |
| Critical point (T, P) | 610.3 K at 35.80 bar |
| insoluble | |
| Solubility | soluble in benzene, acetone, ether |
| Vapor pressure | 5 mmHg |
Refractive index (nD) |
1.3949 |
| Hazards | |
| GHS pictograms |    |
| GHS Signal word | Danger |
GHS hazard statements |
H226, H301, H302 |
| P210, P233, P240, P241, P242, P243, P264, P270, P280, P301+310, P301+312, P303+361+353, P321, P330, P370+378, P403+235, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Flash point | 40 °C (104 °F; 313 K) |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
191 mg/kg fat[1] |
| Related compounds | |
Related alkanenitriles |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Production
Pentanenitrile can be produced by heating 1-chlorobutane with sodium cyanide in dimethyl sulfoxide. This reaction takes about 20 minutes, keeping the temperature below 160 °C. The yield is about 93%.[2]
Another way to get the substance is by heating butyraldehyde with hydroxylamine.[3]
Properties
The pentanenitrile molecule is flexible and can adopt a number of different conformers, so that it will naturally be a mixture. These conformers are called anti-anti (30%), anti-gauche (46%), gauche-anti, gauche-gauche-cis, and gauche-gauche-trans.[5]
Biology
Pentanenitrile is toxic to animals, and produces its action by the liberation of cyanide by cytochrome P450. The cyanide is detoxified and excreted in urine as thiocyanate.[1]
Pentanenitrile is found in Brassica species and varieties such as broccoli.
Pentanenitrile is hydrolyzed to valeric acid by the fungi Gibberella intermedia,[6] Fusarium oxysporum, and Aspergillus niger in which it induces production of the nitrilase enzyme.[7]
References
- Buhler, D. R.; Reed, D. J. (2013). Nitrogen and Phosphorus Solvents. Elsevier. pp. 359–362. ISBN 9781483290201.
- Smiley, Robert; Arnold, Charles (February 1960). "Notes- Aliphatic Nitriles from Alkyl Chlorides". The Journal of Organic Chemistry. 25 (2): 257–258. doi:10.1021/jo01072a600.
- "Red mud catalyzed one-pot synthesis of nitriles from aldehydes and hydroxylamine hydrochloride under microwave irradiation". Arkivoc. 2007 (15): 162. 5 October 2007. doi:10.3998/ark.5550190.0008.f16.
- Toxic Substances Control Act (TSCA) Chemical Substance Inventory. the Office. 1979.
- Crowder, G.A. (October 1989). "Conformational analysis of n-butyl cyanide". Journal of Molecular Structure: THEOCHEM. 200: 235–244. doi:10.1016/0166-1280(89)85056-0.
- Gong, Jin-Song; Li, Heng; Zhu, Xiao-Yan; Lu, Zhen-Ming; Wu, Yan; Shi, Jing-Song; Xu, Zheng-Hong; Yun, Sung-Hwan (30 November 2012). "Fungal His-Tagged Nitrilase from Gibberella intermedia: Gene Cloning, Heterologous Expression and Biochemical Properties". PLoS ONE. 7 (11): e50622. doi:10.1371/journal.pone.0050622. PMC 3511519.

- Kaplan, Ondřej; Vejvoda, Vojtěch; Charvátová-Pišvejcová, Andrea; Martínková, Ludmila (15 August 2006). "Hyperinduction of nitrilases in filamentous fungi". Journal of Industrial Microbiology & Biotechnology. 33 (11): 891–896. doi:10.1007/s10295-006-0161-9.
