Pentane (data page)
This page provides supplementary chemical data on n-pentane.
Material Safety Data Sheet
The handling of this chemical may incur notable safety precautions. It is highly recommend that you seek the Material Safety Datasheet (MSDS) for this chemical from a reliable source such as MSDS Search Engine, and follow its directions.
Structure and properties
| Structure and properties | |
|---|---|
| Index of refraction,[1] nD | 1.3575 at 20 °C |
| Abbe number | ? |
| Dielectric constant,[2] εr | 1.844 ε0 at 20 °C |
| Bond strength | ? |
| Bond length | ? |
| Bond angle | ? |
| Magnetic susceptibility | ? |
| Surface tension[3] | 18.16 dyn/cm at 0 °C 17.03 dyn/cm at 10 °C 15.82 dyn/cm at 20 °C 14.73 dyn/cm at 30 °C 13.66 dyn/cm at 40 °C |
| Viscosity[4] | 0.2894 mPa·s at 0 °C 0.2395 mPa·s at 20 °C 0.2200 mPa·s at 30 °C |
Thermodynamic properties
| Phase behavior | |
|---|---|
| Triple point | 143.46 K (–128.69 °C), 0.076 Pa |
| Critical point | 469.8 K (196.7 °C), 3360 kPa |
| Std enthalpy change of fusion, ΔfusH |
8.4 kJ/mol |
| Std entropy change of fusion, ΔfusS |
58.5 J/(mol·K) |
| Std enthalpy change of vaporization, ΔvapH |
26.200 kJ/mol at 25 °C 25.79 kJ/mol at 36.1 °C |
| Std entropy change of vaporization, ΔvapS |
87.88 J/(mol·K) at 25 °C |
| Solid properties | |
| Std enthalpy change of formation, ΔfH |
? kJ/mol |
| Standard molar entropy, S |
? J/(mol K) |
| Heat capacity, cp | ? J/(mol K) |
| Liquid properties | |
| Std enthalpy change of formation, ΔfH |
–173.5 kJ/mol |
| Standard molar entropy, S |
263.47 J/(mol K) |
| Enthalpy of combustion, ΔcH |
–3509 kJ/mol |
| Heat capacity, cp | 167.19 J/(mol K) at 25 °C |
| Gas properties | |
| Std enthalpy change of formation, ΔfH |
–146.8 kJ/mol |
| Standard molar entropy, S |
347.82 J/(mol K) |
| Enthalpy of combustion, ΔcH |
–3535 kJ/mol |
| Heat capacity, cp | 120.07 J/(mol K) at 25 °C |
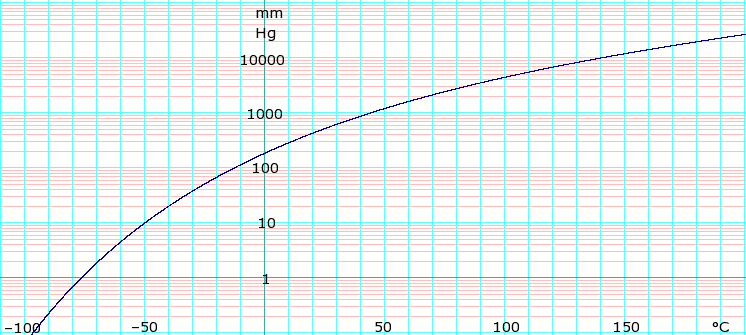
Vapor pressure of liquid
| P in mm Hg | 1 | 10 | 40 | 100 | 400 | 760 | 1520 | 3800 | 7600 | 15200 | 30400 | 45600 | |
| T in °C | –76.6 | –50.1 | –29.2 | –12.6 | 18.5 | 36.1 | 58.0 | 92.4 | 124.7 | 164.3 | — | — | |
Table data obtained from CRC Handbook of Chemistry and Physics 47th ed.
Spectral data
| UV-Vis | |
|---|---|
| λmax | ? nm |
| Extinction coefficient, ε | ? |
| IR | |
| Major absorption bands | 2881, 2940, 2965 cm−1 [6] |
| NMR | |
| Proton NMR | |
| Carbon-13 NMR | |
| Other NMR data | |
| MS | |
| Masses of main fragments |
|
gollark: (although technically there's the osmarks.net status monitor too)
gollark: Simply use exactly one extremely overburdened server, like me.
gollark: I am not accepting feedback on this opinion at the present time.
gollark: Having investigated, I think I side with the fish.
gollark: In other news, I have apparently reached 813 open tabs.
References
- Lange's Handbook of Chemistry, 10th ed. pp. 1234–1237
- Lange's Handbook of Chemistry, 10th ed. pp. 1289–1376
- A.P. Fröba; L. Penedo Pellegrino & A. Leipertz (June 2003). "Viscosity and Surface Tension of Saturated n-Pentane" (PDF). National Institute of Standards and Technology (15th Symposium on Thermophysical Properties). Archived from the original (PDF) on 9 October 2006. Retrieved 30 May 2007.
- Lange's Handbook of Chemistry, 10th ed. pp. 1669–1674
- "Pure Component Properties" (Queriable database). Chemical Engineering Research Information Center. Retrieved 30 May 2007.
- Klingbeil, Adam E.; Jeffries, Jay B.; Hanson, Ronald K. (2007). "Temperature-dependent mid-IR absorption spectra of gaseous hydrocarbons". Journal of Quantitative Spectroscopy and Radiative Transfer. 107 (3): 407–420. doi:10.1016/j.jqsrt.2007.03.004.
Except where noted otherwise, data relate to standard ambient temperature and pressure.
Disclaimer applies.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.