Pentafluorobenzene
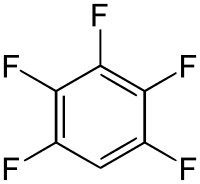
Pentafluorobenzene is an organofluoride compound with the molecular formula C
6HF
5.[1] The compound consists of a benzene ring substituted with five fluorine atoms.[2] The substance is a colorless liquid with a boiling point similar to that of benzene.[3][4] It is prepared by defluorination of highly fluorinated cyclohexanes over hot nickel or iron.[5] Another method involved dehydrofluorination of polyfluorinated cyclohexane using hot aqueous solution of KOH.[6]
 | |
| Names | |
|---|---|
| IUPAC name
1,2,3,4,5-Pentafluorbenzene | |
| Other names
Pentafluorobenzene, phenyl pentafluoride | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.006.054 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C6HF5 | |
| Molar mass | 168.066 g·mol−1 |
| Appearance | Colorless liquid |
| Density | 1.511 g/cm3 |
| Melting point | -47.4 |
| Boiling point | 85 °C (185 °F; 358 K) |
| Insoluble | |
| Hazards | |
| GHS pictograms |    |
| GHS Signal word | Danger |
GHS hazard statements |
H225, H302, H315, H318, H335 |
| Flash point | 14 °C (57 °F; 287 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
References
- "Pentafluorobenzene". Sigma Aldrich. sigmaaldrich.com. Retrieved 8 June 2017.
- "Пентафторбензол" (in Russian). himreakt.ru. Retrieved 8 June 2017.
- "Pentafluorobenzene". NIST. webbook.nist.gov. Retrieved 8 June 2017.
- CRC Handbook of Chemistry and Physics, 90. Edition, CRC Press, Boca Raton, Florida, 2009, ISBN 978-1-4200-9084-0, Section 3, Physical Constants of Organic Compounds, p. 3-414.
- Gething, B.; Patrick, C. R.; Tatlow, J. C.; Banks, R. E.; Barbour, A. K.; Tipping, A. E. (1959). "Thermal Reactions of Highly Fluorinated Cyclo Hexadienes". Nature. 183 (4661): 586–587. Bibcode:1959Natur.183..586G. doi:10.1038/183586a0.
- Nield, E.; Stephens, R.; Tatlow, J. C. (1959). "31. Aromatic polyfluoro-compounds. Part I. The synthesis of aromatic polyfluoro-compounds from pentafluorobenzene". Journal of the Chemical Society (Resumed): 166. doi:10.1039/JR9590000166.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.