PEGylation
PEGylation (often styled pegylation) is the process of both covalent and non-covalent attachment or amalgamation of polyethylene glycol (PEG, in pharmacy called macrogol) polymer chains to molecules and macrostructures, such as a drug, therapeutic protein or vesicle, which is then described as PEGylated (pegylated).[1][2][3] PEGylation is routinely achieved by the incubation of a reactive derivative of PEG with the target molecule. The covalent attachment of PEG to a drug or therapeutic protein can "mask" the agent from the host's immune system (reducing immunogenicity and antigenicity), and increase its hydrodynamic size (size in solution), which prolongs its circulatory time by reducing renal clearance. PEGylation can also provide water solubility to hydrophobic drugs and proteins. Having proven its pharmacological advantages and acceptability, PEGylation technology is the foundation of a growing multibillion-dollar industry.[4]
_alternate.svg.png)
Methodology
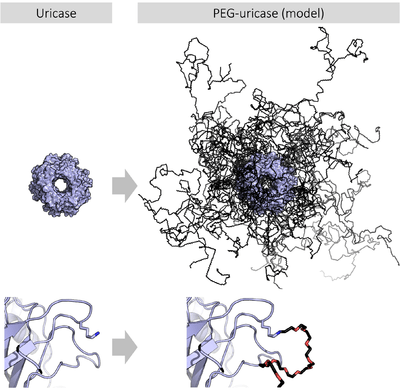
PEGylation is the process of attaching the strands of the polymer PEG to molecules, most typically peptides, proteins, and antibody fragments, that can improve the safety and efficiency of many therapeutics.[6][7] It produces alterations in the physiochemical properties including changes in conformation, electrostatic binding, hydrophobicity etc. These physical and chemical changes increase systemic retention of the therapeutic agent. Also, it can influence the binding affinity of the therapeutic moiety to the cell receptors and can alter the absorption and distribution patterns.
PEGylation, by increasing the molecular weight of a molecule, can impart several significant pharmacological advantages over the unmodified form, such as improved drug solubility, reduced dosage frequency, without diminished efficacy with potentially reduced toxicity, extended circulating life, increased drug stability, and enhanced protection from proteolytic degradation; peglyated forms may also be eligible for patent protection.[8]
PEGylated pharmaceuticals on the market
The attachment of an inert and hydrophilic polymer was first reported around 1970 to extend blood life and control immunogenicity of proteins.[9] Polyethylene glycol was chosen as the polymer.[10][11] In 1981 Davis and Abuchowski founded Enzon, Inc., which brought three PEGylated drugs to market. Abuchowski later founded and is CEO of Prolong Pharmaceuticals.[12]
The clinical value of PEGylation is now well established. ADAGEN (pegademase bovine) manufactured by Enzon Pharmaceuticals, Inc., US was the first PEGylated protein approved by the U.S. Food and Drug Administration (FDA) in March 1990, to enter the market. It is used to treat a form of severe combined immunogenicity syndrome (ADA-SCID), as an alternative to bone marrow transplantation and enzyme replacement by gene therapy. Since the introduction of ADAGEN, a large number of PEGylated protein and peptide pharmaceuticals have followed and many others are under clinical trial or under development stages. Sales of the two most successful products, Pegasys and Neulasta, exceeded $5 billion in 2011.[13][14] All commercially available PEGylated pharmaceuticals contain methoxypoly(ethylene glycol) or mPEG. PEGylated pharmaceuticals on the market (in reverse chronology by FDA approval year) have included:
- Pegvaliase (Biomarin) – PEGylated recombinant phenylalanine ammonia-lyase for the treatment of Phenylketonuria, approved by the FDA for the US in May 2018.[15][16]
- Adynovate – PEGylated Antihemophilic Factor VIII for the treatment of patients with hemophilia A. (Baxalta, 2015)[17]
- Irinotecan liposome (Onivyde) – PEGylated liposomal irinotecan hydrochloride trihydrate for the treatment of metastatic pancreatic cancer in adults proceeding treatment with gemcitabine-based therapy. (Ipsen, 2015)
- Plegridy – PEGylated Interferon Beta-1a for the treatment of patients with relapsing forms of multiple sclerosis. (Biogen, 2014)
- Naloxegol (Movantik) – PEGylated naloxol for the treatment of opioid-induced constipation in adults patients with chronic non-cancer pain (un-pegylated methadone can cause adverse gastrointestinal reactions). (AstraZeneca, 2014)
- Peginesatide (Omontys) – once-monthly medication to treat anemia associated with chronic kidney disease in adult patients on dialysis (Affymax/Takeda Pharmaceuticals, 2012)
- Pegloticase (Krystexxa) – PEGylated uricase for the treatment of gout (Savient, 2010)
- Certolizumab pegol (Cimzia) – monoclonal antibody for treatment of moderate to severe rheumatoid arthritis and Crohn's disease, an inflammatory gastrointestinal disorder (Nektar/UCB Pharma, 2008)
- Methoxy polyethylene glycol-epoetin beta (Mircera) – PEGylated form of erythropoietin to combat anemia associated with chronic kidney disease (Roche, 2007)
- Pegaptanib (Macugen) – used to treat neovascular age-related macular degeneration (Pfizer, 2004)
- Pegfilgrastim (Neulasta) – PEGylated recombinant methionyl human granulocyte colony-stimulating factor for severe cancer chemotherapy-induced neutropenia (Amgen, 2002)
- Pegvisomant (Somavert) – PEG-human growth hormone mutein antagonist for treatment of Acromegaly (Pfizer, 2002)
- Peginterferon alfa-2a (Pegasys) – PEGylated interferon alpha for use in the treatment of chronic hepatitis C and hepatitis B (Hoffmann-La Roche, 2002)
- Peginterferon alfa-2b (PegIntron) – PEGylated interferon alpha for use in the treatment of chronic hepatitis C and hepatitis B (Schering-Plough/Enzon, 2000)
- Doxorubicin HCl liposome (Doxil/Caelyx) – PEGylated liposome containing doxorubicin for the treatment of cancer (Alza, 1995)
- Pegaspargase (Oncaspar) – PEGylated L-asparaginase for the treatment of acute lymphoblastic leukemia in patients who are hypersensitive to the native unmodified form of L-asparaginase (Enzon, 1994). This drug was recently approved for front line use.
- Pegademase bovine (Adagen) – PEG-adenosine deaminase for the treatment of severe combined immunodeficiency disease (SCID) (Enzon, 1990)
Use in research
PEGylation has practical uses in biotechnology for protein delivery, cell transfection, and gene editing in non-human cells.[18]
Process
The first step of the PEGylation is the suitable functionalization of the PEG polymer at one or both ends. PEGs that are activated at each end with the same reactive moiety are known as "homobifunctional", whereas if the functional groups present are different, then the PEG derivative is referred as "heterobifunctional" or "heterofunctional". The chemically active or activated derivatives of the PEG polymer are prepared to attach the PEG to the desired molecule.[19]
The overall PEGylation processes used to date for protein conjugation can be broadly classified into two types, namely a solution phase batch process and an on-column fed-batch process.[20] The simple and commonly adopted batch process involves the mixing of reagents together in a suitable buffer solution, preferably at a temperature between 4 and 6 °C, followed by the separation and purification of the desired product using a suitable technique based on its physicochemical properties, including size exclusion chromatography (SEC), ion exchange chromatography (IEX), hydrophobic interaction chromatography (HIC) and membranes or aqueous two phase systems.[21][22]
The choice of the suitable functional group for the PEG derivative is based on the type of available reactive group on the molecule that will be coupled to the PEG. For proteins, typical reactive amino acids include lysine, cysteine, histidine, arginine, aspartic acid, glutamic acid, serine, threonine and tyrosine. The N-terminal amino group and the C-terminal carboxylic acid can also be used as a site specific site by conjugation with aldehyde functional polymers.[23]
The techniques used to form first generation PEG derivatives are generally reacting the PEG polymer with a group that is reactive with hydroxyl groups, typically anhydrides, acid chlorides, chloroformates and carbonates. In the second generation PEGylation chemistry more efficient functional groups such as aldehyde, esters, amides etc. made available for conjugation.
As applications of PEGylation have become more and more advanced and sophisticated, there has been an increase in need for heterobifunctional PEGs for conjugation. These heterobifunctional PEGs are very useful in linking two entities, where a hydrophilic, flexible and biocompatible spacer is needed. Preferred end groups for heterobifunctional PEGs are maleimide, vinyl sulfones, pyridyl disulfide, amine, carboxylic acids and NHS esters.
Third generation pegylation agents, where the polymer has been branched, Y shaped or comb shaped are available and show reduced viscosity and lack of organ accumulation.[24]
Limitations
Unpredictability in clearance times for PEGylated compounds may lead to the accumulation of large molecular weight compounds in the liver leading to inclusion bodies with no known toxicologic consequences.[25] Furthermore, alteration in the chain length may lead to unexpected clearance times in vivo.[26] Moreover, the experimental conditions of PEGylation reaction (i.e. pH, temperature, reaction time, overall cost of the process and molar ratio between PEG derivative and peptide) also have an impact on the stability of the final PEGylated products[27]. To overcome the above mentioned limitations different strategies such as changing the size (Mw), the number, the location and the type of linkage of PEG molecule were offered by several researchers[28]
References
- Jokerst, J. V.; Lobovkina, T.; Zare, R. N.; Gambhir, S. S. (2011). "Nanoparticle PEGylation for imaging and therapy". Nanomedicine. 6 (4): 715–728. doi:10.2217/nnm.11.19. PMC 3217316. PMID 21718180.CS1 maint: uses authors parameter (link)
- Knop, K.; Hoogenboom, R.; Fischer, D.; Schubert, U. S. (2010). "Poly(ethylene glycol) in Drug Delivery: Pros and Cons as Well as Potential Alternatives". Angew. Chem. Int. Ed. 49 (36): 6288–6308. doi:10.1002/anie.200902672. PMID 20648499.CS1 maint: uses authors parameter (link)
- Veronese, F. M.; Mero, A. (2008). "The impact of PEGylation on biological therapies". BioDrugs. 22 (5): 315–329. doi:10.2165/00063030-200822050-00004. PMID 18778113.CS1 maint: uses authors parameter (link)
- Damodaran V. B. ; Fee C. J. (2010). "Protein PEGylation: An overview of chemistry and process considerations". European Pharmaceutical Review. 15 (1): 18–26.
- Sherman, MR; Saifer, MG; Perez-Ruiz, F (3 January 2008). "PEG-uricase in the management of treatment-resistant gout and hyperuricemia". Advanced Drug Delivery Reviews. 60 (1): 59–68. doi:10.1016/j.addr.2007.06.011. PMID 17826865.
- Veronese, F. M.; Harris, J. M. (2002). "Introduction and overview of peptide and protein pegylation". Advanced Drug Delivery Reviews. 54 (4): 453–456. doi:10.1016/S0169-409X(02)00020-0. PMID 12052707.
- Porfiryeva, N. N.; Moustafine, R. I.; Khutoryanskiy, V. V. (2020-01-01). "PEGylated Systems in Pharmaceutics". Polymer Science, Series C. 62 (1): 62–74. doi:10.1134/S181123822001004X. ISSN 1555-614X.
- Milla, P; Dosio, F (13 January 2012). "PEGylation of proteins and liposomes: a powerful and flexible strategy to improve the drug delivery". Current Drug Metabolism. 13 (1): 105–119. doi:10.2174/138920012798356934. hdl:2318/86788. PMID 21892917.
- Davis, Frank F. (2002). "The origin of pegnology". Advanced Drug Delivery Reviews. 54 (4): 457–8. doi:10.1016/S0169-409X(02)00021-2. PMID 12052708.
- Abuchowski, A; Van Es, T; Palczuk, N. C.; Davis, F. F. (1977). "Alteration of immunological properties of bovine serum albumin by covalent attachment of polyethylene glycol". The Journal of Biological Chemistry. 252 (11): 3578–81. PMID 405385.
- Abuchowski, A; McCoy, J. R.; Palczuk, N. C.; Van Es, T; Davis, F. F. (1977). "Effect of covalent attachment of polyethylene glycol on immunogenicity and circulating life of bovine liver catalase". The Journal of Biological Chemistry. 252 (11): 3582–6. PMID 16907.
- "Dr. Abraham Abuchowski, Ph.D. – Home". prolongpharma.com. Retrieved 2020-01-15.
- Klauser, Alexander (Head), Roche Group Media Relations, "Roche in 2011: Strong results and positive outlook," www.roche.com/med-cor-2012-02-01-e.pdf, Feb 1, 2012, p.7
- "Amgen 2011 Annual Report and Financial Summary," 2011 AnnualReport.pdf, Feb 23 2012, p. 71
- Powers, Marie (May 29, 2018). "Biomarin aces final exam: Palynziq gains FDA approval to treat PKU in adults". BioWorld.
- Levy, Harvey L.; Sarkissian, Christineh N.; Stevens, Raymond C.; Scriver, Charles R. (June 2018). "Phenylalanine ammonia lyase (PAL): From discovery to enzyme substitution therapy for phenylketonuria". Molecular Genetics and Metabolism. 124 (4): 223–229. doi:10.1016/j.ymgme.2018.06.002. PMID 29941359.
- https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm472643.htm
- Balazs, Daniel; Godbey, WT (29 October 2010). "Liposomes for Use in Gene Delivery". Journal of Drug Delivery. 2011: 326497. doi:10.1155/2011/326497. PMC 3066571. PMID 21490748.
- Pasut, G.; Veronese, F. M. (2012). "State of the art in PEGylation: The great versatility achieved after forty years of research". Journal of Controlled Release. 161 (2): 461–472. doi:10.1016/j.jconrel.2011.10.037. PMID 22094104.CS1 maint: uses authors parameter (link)
- Fee, Conan J.; Van Alstine, James M. (2006). "PEG-proteins: Reaction engineering and separation issues". Chemical Engineering Science. 61 (3): 924. CiteSeerX 10.1.1.509.2865. doi:10.1016/j.ces.2005.04.040.
- Veronese, edited by Francesco M. (2009). "Protein conjugates purification and characterization". PEGylated protein drugs basic science and clinical applications (Online-Ausg. ed.). Basel: Birkhäuser. pp. 113–125. ISBN 978-3-7643-8679-5.CS1 maint: extra text: authors list (link)
- Fee, Conan J. (2003). "Size-exclusion reaction chromatography (SERC): A new technique for protein PEGylation". Biotechnology and Bioengineering. 82 (2): 200–6. doi:10.1002/bit.10561. hdl:10092/351. PMID 12584761.
- Fee, Conan J.; Damodaran, Vinod B. (2012). Production of PEGylated Proteins. Biopharmaceutical Production Technology. p. 199. doi:10.1002/9783527653096.ch7. ISBN 9783527653096.
- Ryan, Sinéad M; Mantovani, Giuseppe; Wang, Xuexuan; Haddleton, David M; Brayden, David J (2008). "Advances in PEGylation of important biotech molecules: Delivery aspects". Expert Opinion on Drug Delivery. 5 (4): 371–83. doi:10.1517/17425247.5.4.371. PMID 18426380.
- Kawai, F (2002). "Microbial degradation of polyethers". Applied Microbiology and Biotechnology. 58 (1): 30–8. doi:10.1007/s00253-001-0850-2. PMID 11831473.
- Veronese, F. M. (2001). "Peptide and protein PEGylation: A review of problems and solutions". Biomaterials. 22 (5): 405–17. doi:10.1016/s0142-9612(00)00193-9. PMID 11214751.
- J. González-Valdez, M. Rito-Palomares, J. Benavides, Advances and trends in the design, analysis, and characterization of polymer–protein conjugates for “PEGylaided” bioprocesses, Anal. Bioanal. Chem. 403 (2012) 2225–2235. doi:10.1007/s00216-012-5845-6.
- G. Zhang, B. Han, X. Lin, X. Wu, H. Yan, Modification of Antimicrobial Peptide with Low Molar Mass Poly(ethylene glycol), J. Biochem. (Tokyo). 144 (2008) 781–788. doi:10.1093/jb/mvn134. [19] S. Obuobi, Y. Wang, J.S. Khara, A. Riegger, S.L. Kuan, P.L.R. Ee, Antimicrobial and Anti-Biofilm Activities of Surface Engineered Polycationic Albumin Nanoparticles with Reduced Hemolytic Activity, Macromol. Biosci. 18 (2018) 1800196. doi:10.1002/mabi.201800196.